Ichneumonopsis burmensis Hardy, 1973
|
publication ID |
https://doi.org/ 10.5852/ejt.2017.317 |
|
DOI |
https://doi.org/10.5281/zenodo.3846691 |
|
persistent identifier |
https://treatment.plazi.org/id/821687F9-FFB0-674F-FDE6-84F6AD52485B |
|
treatment provided by |
Carolina |
|
scientific name |
Ichneumonopsis burmensis Hardy, 1973 |
| status |
|
Ichneumonopsis burmensis Hardy, 1973 View in CoL
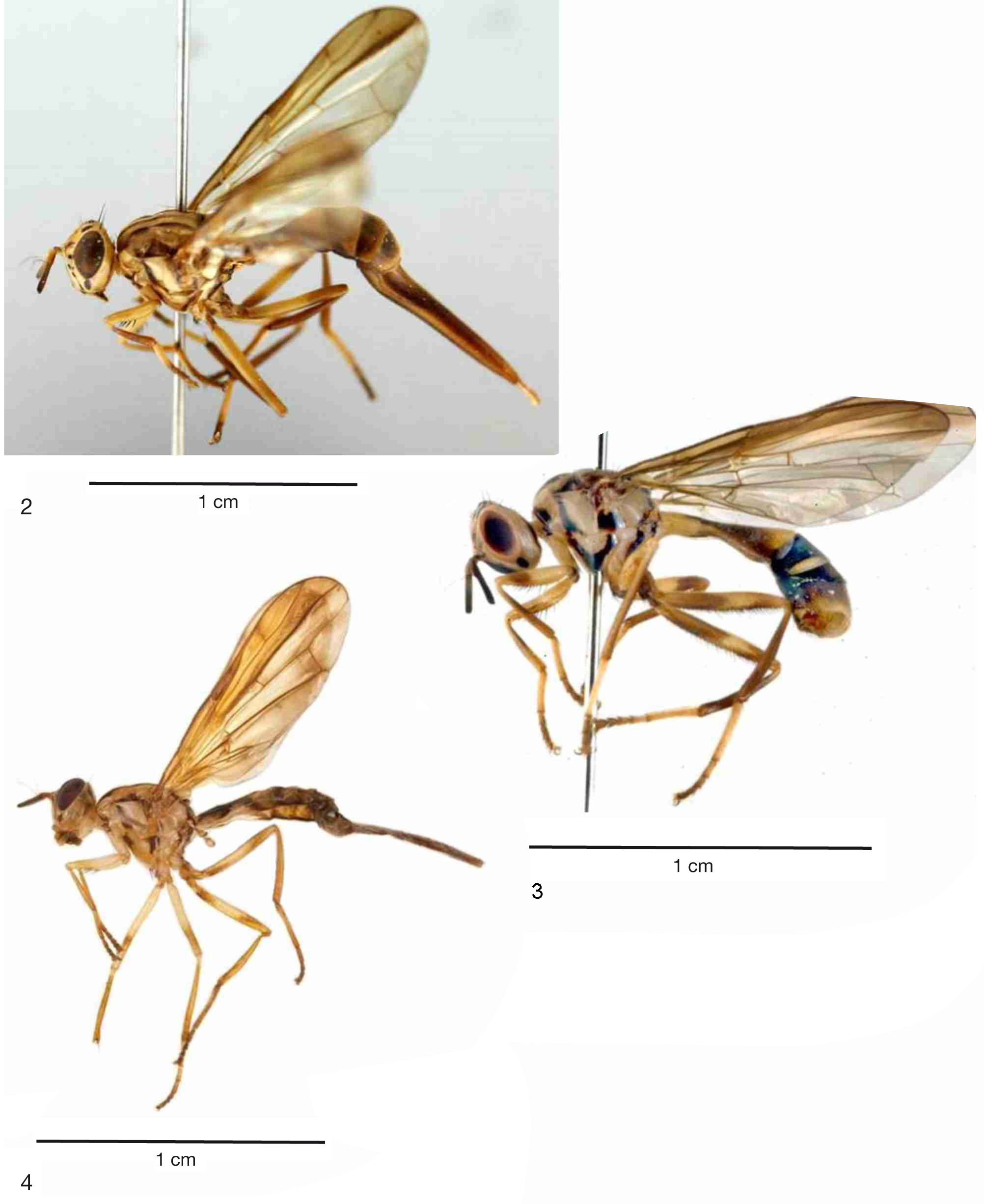
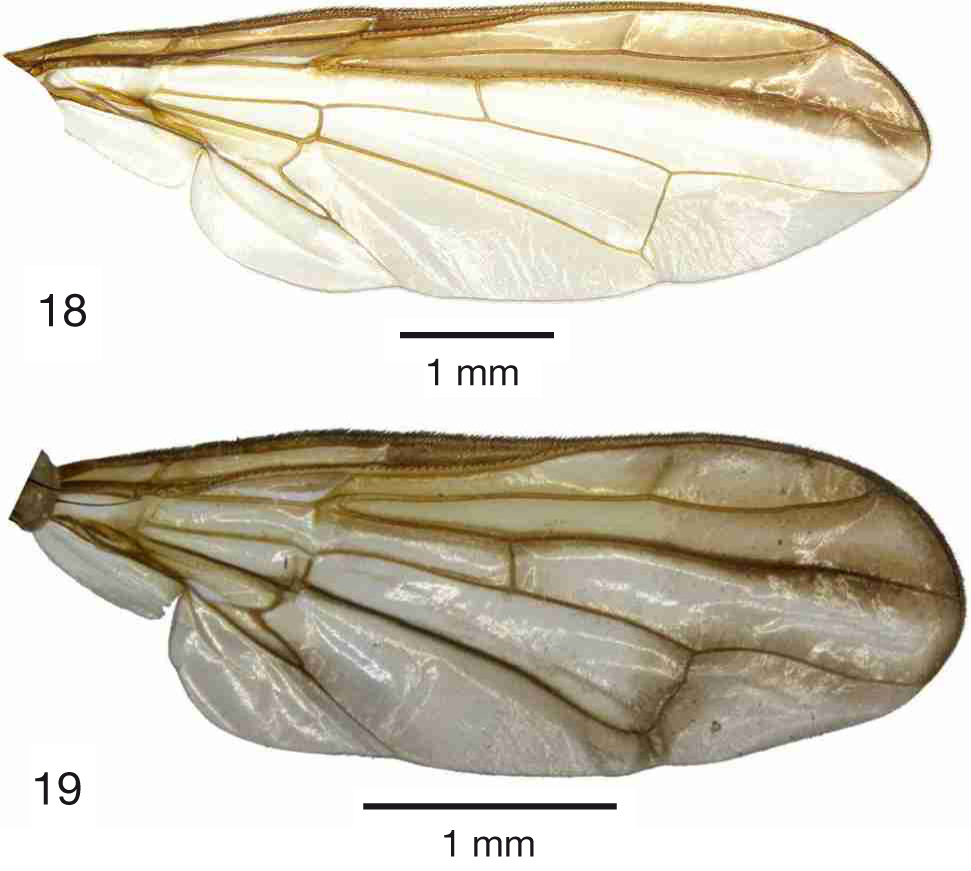
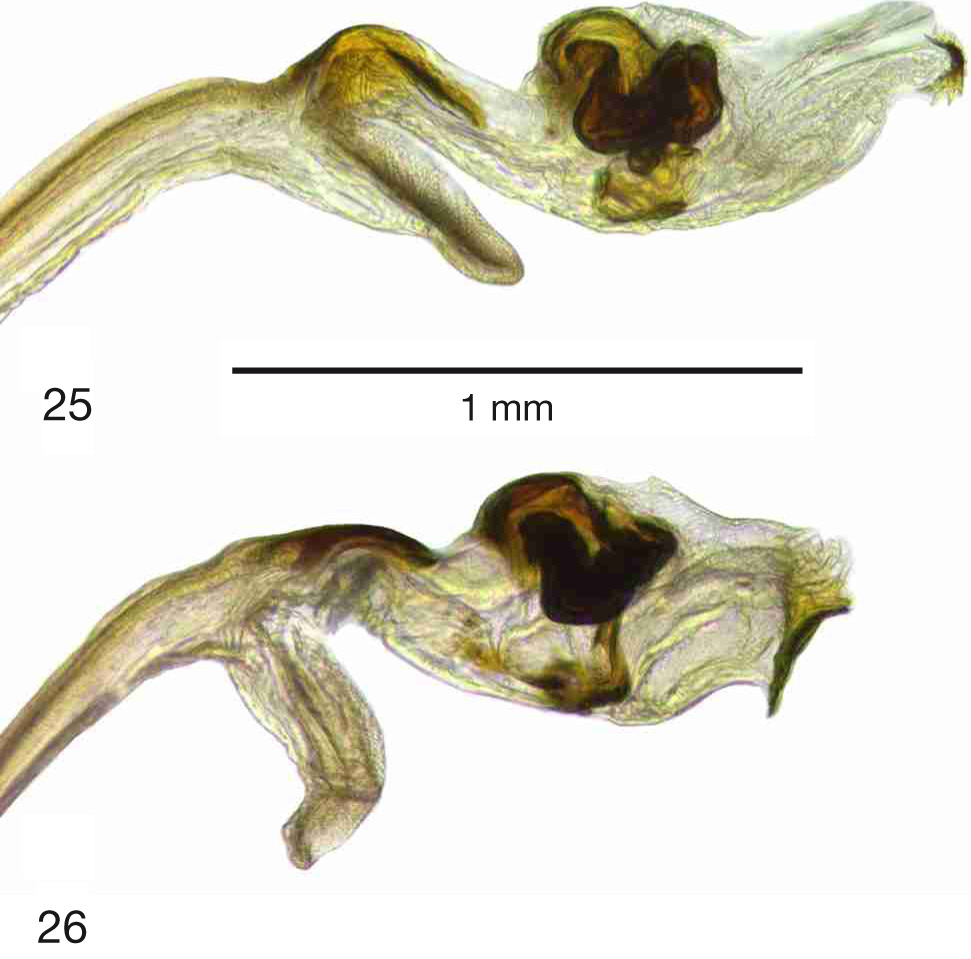
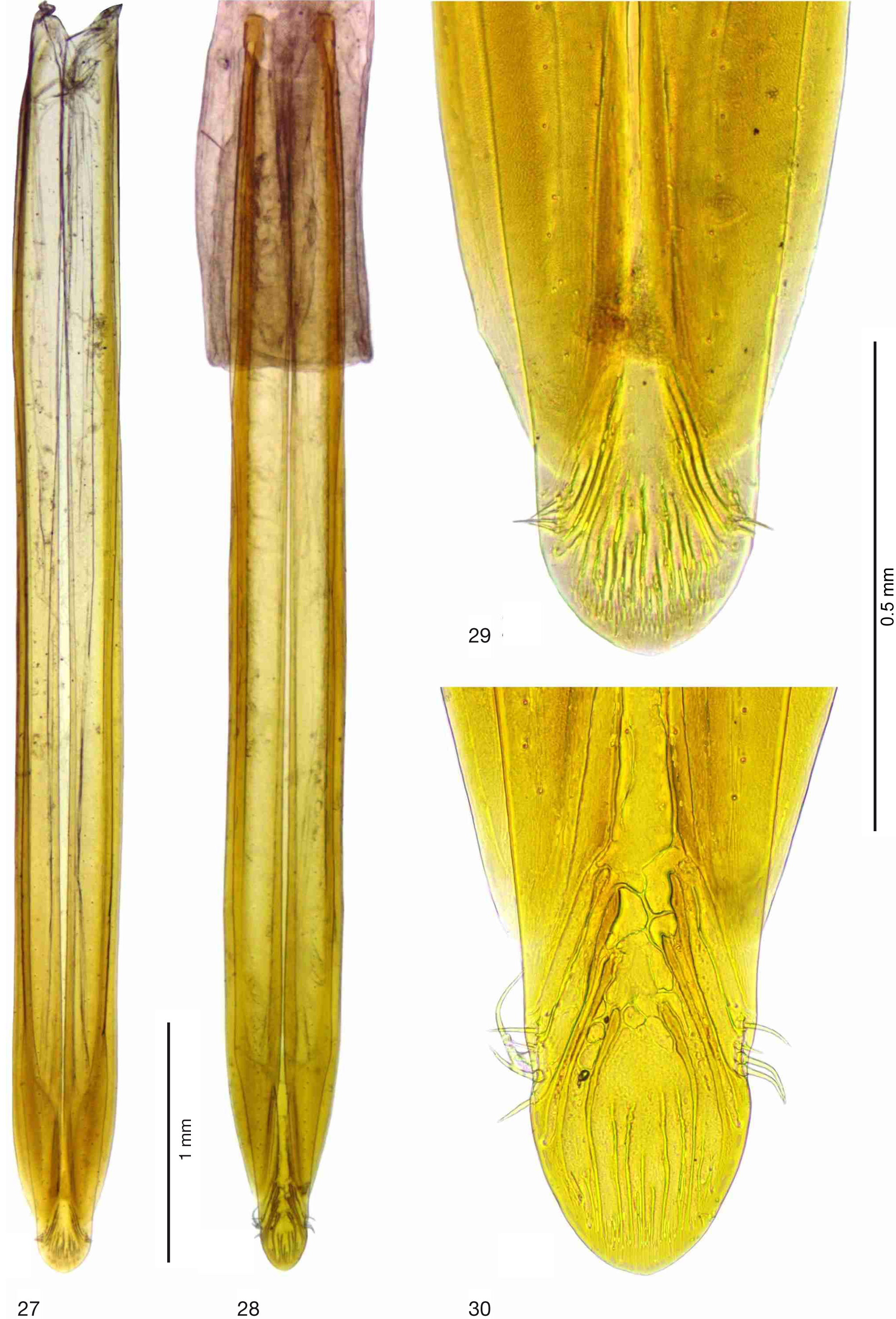
Figs 1–2 View Fig View Figs 2–4 , 7 View Figs 7–9 , 10 View Figs 10–12 , 13 View Figs 13–17 , 18 View Figs 18–19 , 20–23 View Figs 20–21 View Figs 22–24 , 25 View Figs 25–26 , 27 View Figs 27–30 , 31–34 View Figs 31–34
Ichneumonopsis burmensis Hardy, 1973: 133 View in CoL .
Ichneumonopsis burmensis View in CoL – Hardy 1986: 54, 56 (key). — Drew & Hancock 1994: 830. — Norrbom et al. 1999b: 161. — Radhakrishnan 2000: 203 (first record for India). — Kovac et al. 2006: 184. — Kovac et al. 2013: 117 View Cited Treatment (biology and immature stages).
Material examined
MYANMAR: holotype, ♂, Mt. Victoria, Chin Hills , 1400 m, Apr. 1938, G. Heinrich leg. ( BMNH); allotype, ♀, same data as holotype ( BMNH).
INDIA: 1 ♀, Manipur, Churachandpur, 915 m, 10 May 1976, S. Biswas leg., no. B 44, Ghorpade Collection Bangalore, purchased by Tel Aviv University, 2002 ( SMNHTAU); 1 ♂, 2 ♀♀, Meghalaya, Nongpoh Forest, 25–28 Apr. 1980, A. Freidberg leg. ( SMNHTAU).
THAILAND: all specimens were reared by D. Kovac from bamboo shoots of Pseudoxytenanthera albociliata , collected in North Thailand, Mae Hong Son, Pangmapha, near Ban Nam Rin, all deposited in SMF or SMNHTAU: larva collected 1 Dec. 2008, adult 22 May 2009 (1 ♂); larva collected Nov. 2008, adult 16 May 2009 (1 ♂, sample 12); alk 29, probe 13, larva collected 21 Nov. 2008, puparium 27 Jan. 2008, adult 17 Mar. 2009 (1 ♀, sample 29); larva collected 31 Nov. 2008, adult 17 Mar. 2009 (1 ♀, sample 2); larva collected Nov. 2008, adult 29 May 2009 (1 ♂, sample 13); larva collected 21 Nov. 2008, adult 29 May 2009 (1 ♂, sample 14); larva collected 21 Nov. 2008, adult 29 May 2009 (1 ♂, sample 14); larva collected 15 Nov. 2009, puparium 21 Dec. 2009, adult 7 Apr. 2010 (1 ♂, Z56/1/09); larva collected 15 Nov. 2009, adult 30 Apr. 2010 (1 ♂, Z49/2/09); larva collected 15 Nov. 2009, puparium 17 Dec. 2009, adult 30 Apr. 2010 (1 ♀, Z55/1/09); larvae collected 28 Nov. 2009, adults 3 May 2010 (1 ♂, 1 ♀, Z57/2/09); larva collected 28 Nov. 2009, adult 7 May 2010 (1 ♂, Z57/3/09); larva collected 15 Nov. 2012, adult 11 May 2013 (1 ♀, Z68/2/12b).
Redescription
Hardy’s (1973) original description is mostly adequate, requiring only a few comments and a description of the male terminalia (which is lacking in the original description).
Head ( Fig. 7 View Figs 7–9 )
COLOURATION. Number of lateral facial spots varies between 2 ( Hardy 1973: 133, fig. 58b) and 4, spot pattern sometimes asymmetrical; parafacial sometimes with small dark spot in addition to large dorsal spot.
CHAETOTAXY. Tiny frontal seta or enlarged setula sometimes present (more so in females) slightly dorsal to large parafacial spot.
Thorax ( Figs 7 View Figs 7–9 , 10 View Figs 10–12 )
COLOURATION. Scutal pattern ( Fig. 7 View Figs 7–9 ) comprised of vittae best developed in dark specimens (from Thailand): lateral (notopleural) vitta (pair) entirely blackish or brownish centrally; dorsocentral vitta (pair) extending from anterior brown margin of postpronotum as nearly complete vitta (narrowly but distinctly interrupted at transverse suture) to scutoscutellar suture, and both these vittae connect by blackish transverse band along this suture; median vitta (single) narrower and paler than dorsocentral vitta, extending from scapular setae to scutoscutellar suture, also connecting to scutoscutellar band; (pair of) complete yellow submedian bands result, extending from anterior margin of scutum to blackish scutoscutellar band, each about as wide as dorsocentral vitta. In paler specimens (from India) dark bands much paler and reduced, mostly less contrasted with yellow background, although brownishyellow lateral vitta well contrasted with adjacent yellow areas, and narrow median vitta visible throughout its length to or almost to transverse (scutoscutellar) band. Pleural pattern ( Fig. 10 View Figs 10–12 ; best developed in dark specimens) comprised of black vertical or oblique bands and/or spots on whitish or yellowish background as follows: proepisternum black, black extending dorsally to dark area anterior to postpronotum, ventrally to proepimeron (not entirely black), and further “extending” ventrally onto forecoxa dorsobasally; anepisternum with large triangular stripe extending from posterior end of postpronotum and notopleuron to anteroventral corner of anepisternum, just beyond delicate suture extending from near this corner to anepisternal seta; katepisternum with wide “v”-like black pattern, covering most of sclerite except dorsomedially and ventrally where yellowish; posterior arm of “v” “extending over anepimeron, including greater ampulla; narrow sinuous black band extending vertically along posterior half of meron, around anterodorsal margin of spiracle, penetrating into ventral margin of katatergite and reaching base of halter. Postcoxal bridge sclerotized and black. In specimens from India dark pleural pattern mostly brown or dark yellow. Scutellum whitish, narrowly darkened (brownish or blackish) basally; subscutellum pale or dark yellowish, with brownish or blackish lateral and dorsal margins. Calypteres short, slightly bulging anterolaterally.
Legs ( Fig. 13 View Figs 13–17 )
As for genus.
Wing ( Fig. 18 View Figs 18–19 )
Pattern mostly as in Hardy (1973: 133, fig. 58d), with following additions: dark pattern along and posterior to last section of vein R 4+5 distinctly darker than adjacent parts of pattern; cell bcu and surrounding yellowish.
Abdomen ( Figs 20–21 View Figs 20–21 )
TERMINALIA, MALE ( Figs 22–23 View Figs 22–24 , 25 View Figs 25–26 ). Digitiform prolongation of surstylus about four times as long as wide; setae on epandrium conspicuous, similar in appearance and density to those on proctiger; nonsclerotized part of glans beyond cochleate sclerotization elongate, about 4 times as long as wide, apically with small sclerotized sclerite about 0.25–0.30 times as long as width of cochleate complex.
TERMINALIA, FEMALE ( Figs 27–29 View Figs 27–30 ). Cercal unit relatively broad, without constriction, with system of longitudinal “canals” crowded toward tip (tip of aculeus illustrated by Hardy 1973: fig. 58e); sensory setae short, about 0.12–0.16 times as long as width of aculeus at this level; spermathecae illustrated and described by Hardy (1973: fig. 58c), who wrote: “Two small round spermathecae present”. The spermathecae, however, were not found in our dissections.
Measurements (length, in mm)
Male: body: 11.5–13.5; wing: 10.0–10.6; female: body, including oviscape: 18.0–24.1; wing: 10.5–11.8; oviscape: 6.9–8.5.
Biology and immature stages ( Figs 31–34 View Figs 31–34 )
A detailed biological account of this species was the subject of another publication ( Kovac et al. 2013). Only the following few relevant comments have been taken from that publication in order to add some perspective to the present one. In north-west Thailand I. burmensis larvae were found to develop in shoots of the bamboo Pseudoxytenanthera albociliata (Munro) Nguyen ( Fig. 31 View Figs 31–34 ). In our study area the bamboo clumps of P. albociliata grew in open clearings, at the edges of fields, or in secondary forest at altitudes of 600–1200 m. In addition, we have one, newly reared specimen of I. burmensis from a side branch of a bamboo shoot of Dendrocalamus strictus (Roxburgh) Nees.
Each infested bamboo shoot usually contained only one internode inhabited by a single I. burmensis larva ( Fig. 33 View Figs 31–34 ) which pupariated in a cocoon ( Fig. 34 View Figs 31–34 ). The infested internode was the 4 th– 6 th internode below the apex. The larvae fed mainly in the lower half of the internode on the white pith found in the internode cavity, and also damaged the bamboo wall at the base of the internode. Due to these feeding activities the apical 4–5 internodes of the bamboo shoot died off and fell to the ground, with the infested internode remaining at the apex of the bamboo shoot ( Fig. 32 View Figs 31–34 ).
The fully-grown larvae usually moved to the base of the internode cavity, where they created a cocoon by tearing off strips of vascular bundles from the bamboo wall ( Fig. 33 View Figs 31–34 ) and enclosing themselves using finer bamboo particles probably mixed with frass. The larvae pupariated in the cocoon ( Fig. 34 View Figs 31–34 ) and remained there for about three months during the dry season. During that time the upper two-thirds of the infested internode broke off and fell to the ground, but the puparium usually remained in place at the tip of the bamboo shoot. However, sometimes the larvae pupariated in the upper part of the internode and then also fell with it to the ground still enclosed by the internode cavity and the cocoon.
The larvae of I. burmensis were found in the field in October and November. They pupariated in November or December and the adults emerged between the end of March and beginning of May. Most (and the largest) shoots of P. albociliata appeared between September and November, but smaller shoots also appeared after the start of the rainy season in June and July. Combining the phenological data obtained in India and Thailand, it appears that I. burmensis is bivoltine, since the adults were collected or reared from larvae during two distinct seasons, i.e., March – May and October – November.
The bamboo microhabitat of I. burmensis is similar to that of the Gastrozonini Anoplomus rufipes Hardy, 1973 . Larvae of A. rufipes also colonized a single internode of a living bamboo shoot, namely the 5 th– 6 th internode below the apex ( Kovac 2015). However, they generally inhabited a larger bamboo species, Cephalostachyum pergracile Munro , and there the infested internodes contained up to 40 larvae. Unlike A. rufipes and other Gastrozonini , the larvae of I. burmensis did not skip and they pupariated in the internode and not in the soil (thereby removing the need to skip).
Distribution
India (Meghalaya, Manipur), Myanmar and Thailand (Mae Hong Son).
Comments
Ichneumonopsis burmensis was known from eastern India (Meghalaya, Manipur: Drew & Hancock 1994, Radhakrishnan 2000), western Myanmar ( Hardy, 1973) and northwest Thailand ( Kovac et al. 2013 and the present publication). It is probably more widespread than indicated, and we assume that it occurs at least in countries where the associated bamboo species grows, i.e., in Nepal, India, Bangladesh, Myanmar (eastern and southern parts), Thailand, Laos and Vietnam ( Ohrenberger 1999). The Thai population is darker than the Indian population.
| BMNH |
United Kingdom, London, The Natural History Museum [formerly British Museum (Natural History)] |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Dacinae |
|
Tribe |
Gastrozonini |
|
Genus |
Ichneumonopsis burmensis Hardy, 1973
| Freidberg, Amnon, Kovac, Damir & Shiao, Shiuh-Feng 2017 |
Ichneumonopsis burmensis
| Kovac D. & Freidberg A. & Steck G. 2013: 117 |
| Kovac D. & Dohm P. & Freidberg A. & Norrbom A. L. 2006: 184 |
| Radhakrishnan C. 2000: 203 |
| Norrbom A. L. & Carroll L. E. & Thompson F. C. & White I. M. & Freidberg A. 1999: 161 |
| Drew R. A. I. & Hancock D. L. 1994: 830 |
| Hardy D. E. 1986: 54 |
Ichneumonopsis burmensis
| Hardy D. E. 1973: 133 |