Meropsiella Conci, 1941
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4313.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A5Fdfba5-F992-44A8-84C2-1756C943C19B |
|
DOI |
https://doi.org/10.5281/zenodo.5297057 |
|
persistent identifier |
https://treatment.plazi.org/id/832187E9-FE94-FEDC-FF74-6298FCD8FB3C |
|
treatment provided by |
Plazi |
|
scientific name |
Meropsiella Conci, 1941 |
| status |
|
Nirmus Nitzsch, 1818: 291 (in partim).
Degeeriella Neumann, 1906: 60 (in partim). Brueelia Kéler, 1936a: 257 (in partim).
Type host. Nirmus apiastri Denny, 1842: 52 , by original designation.
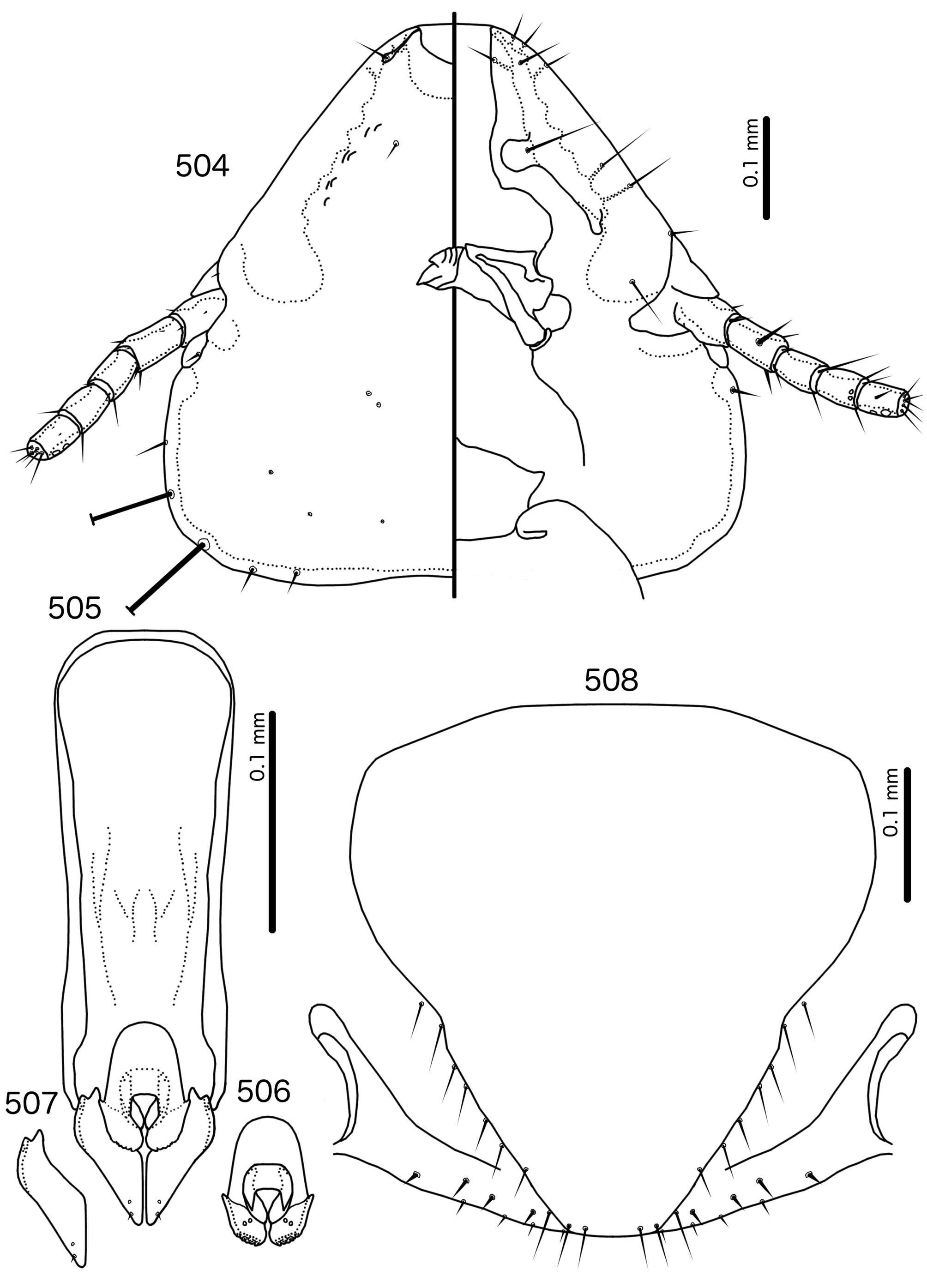
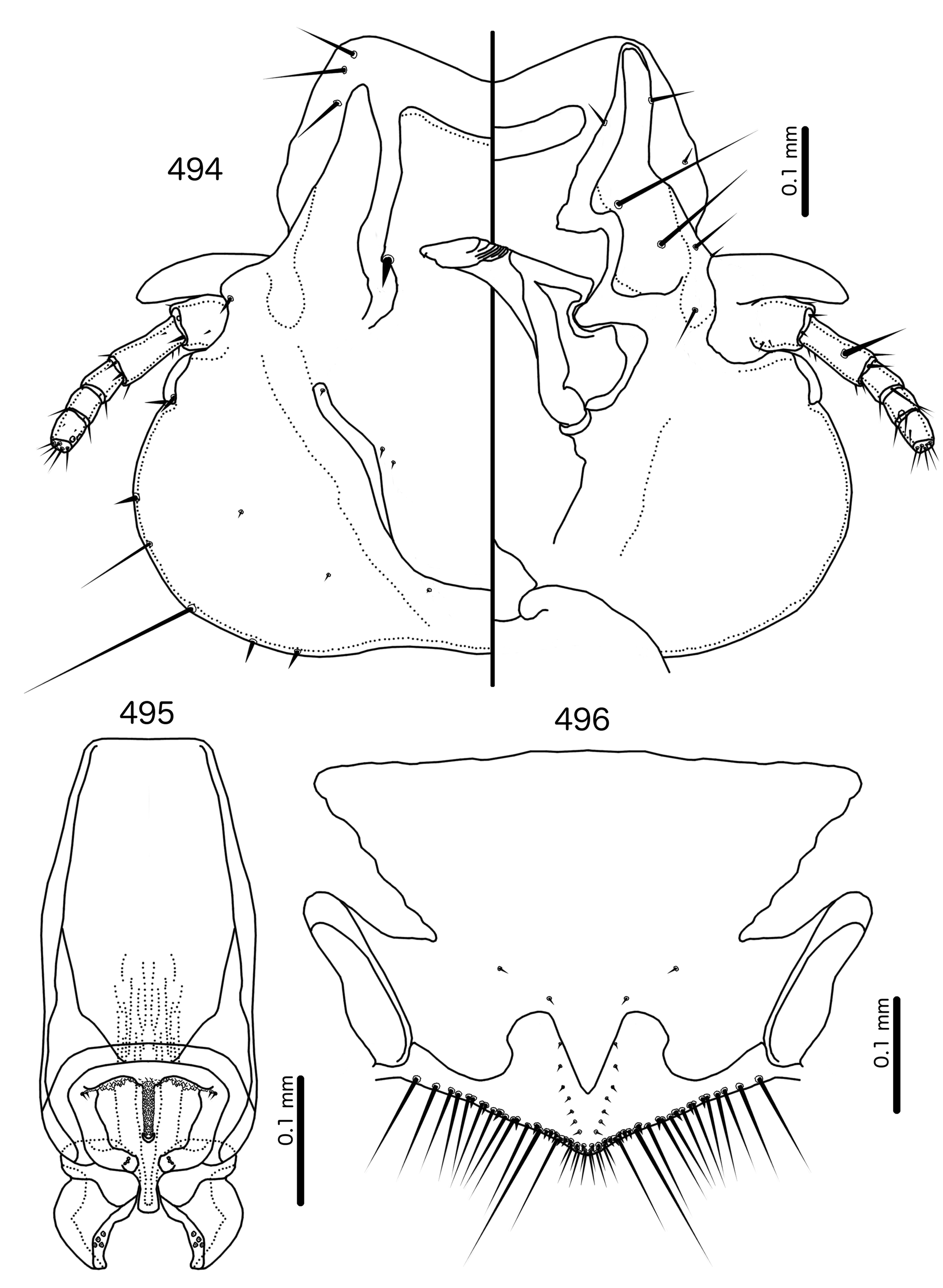
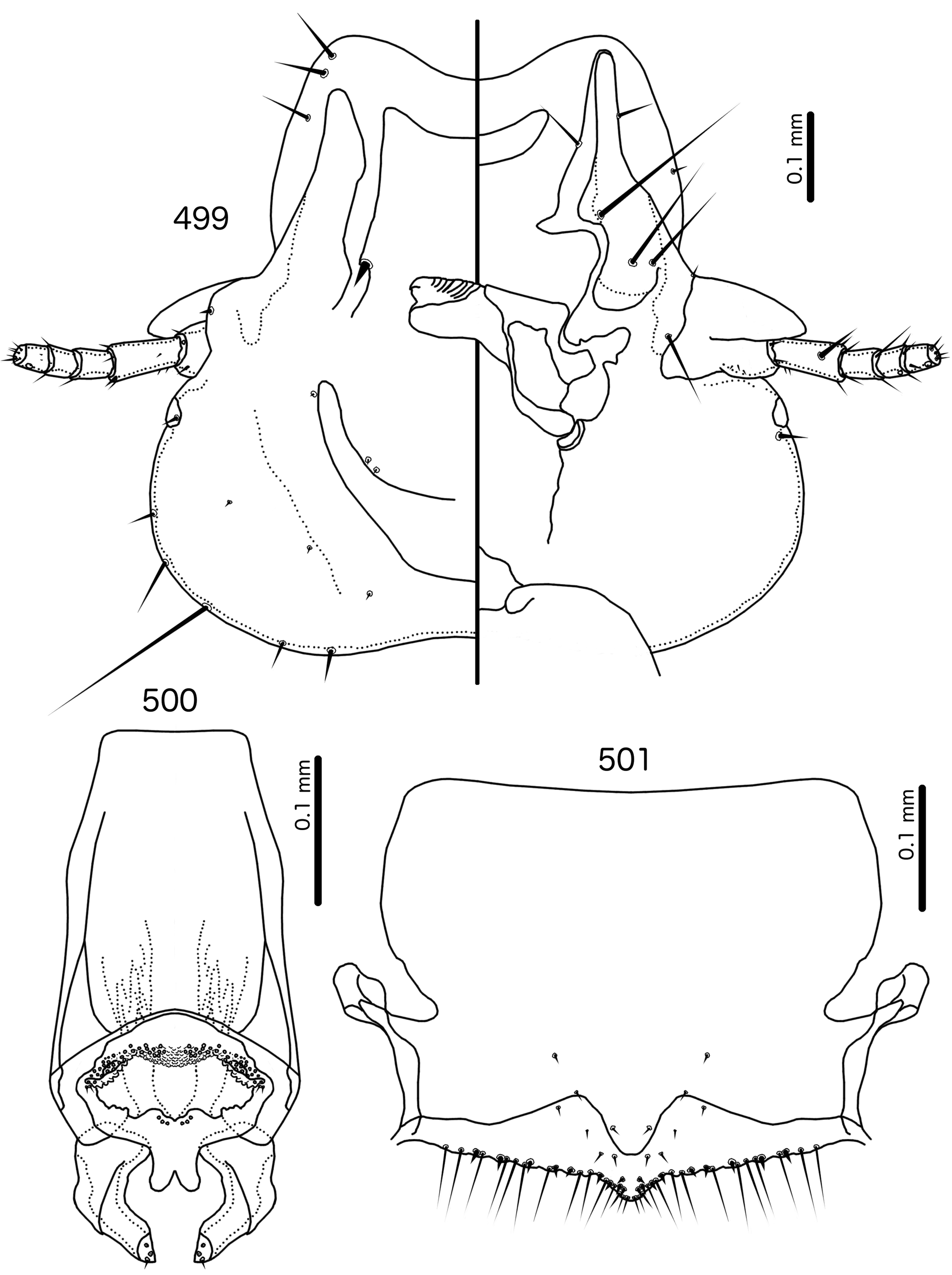
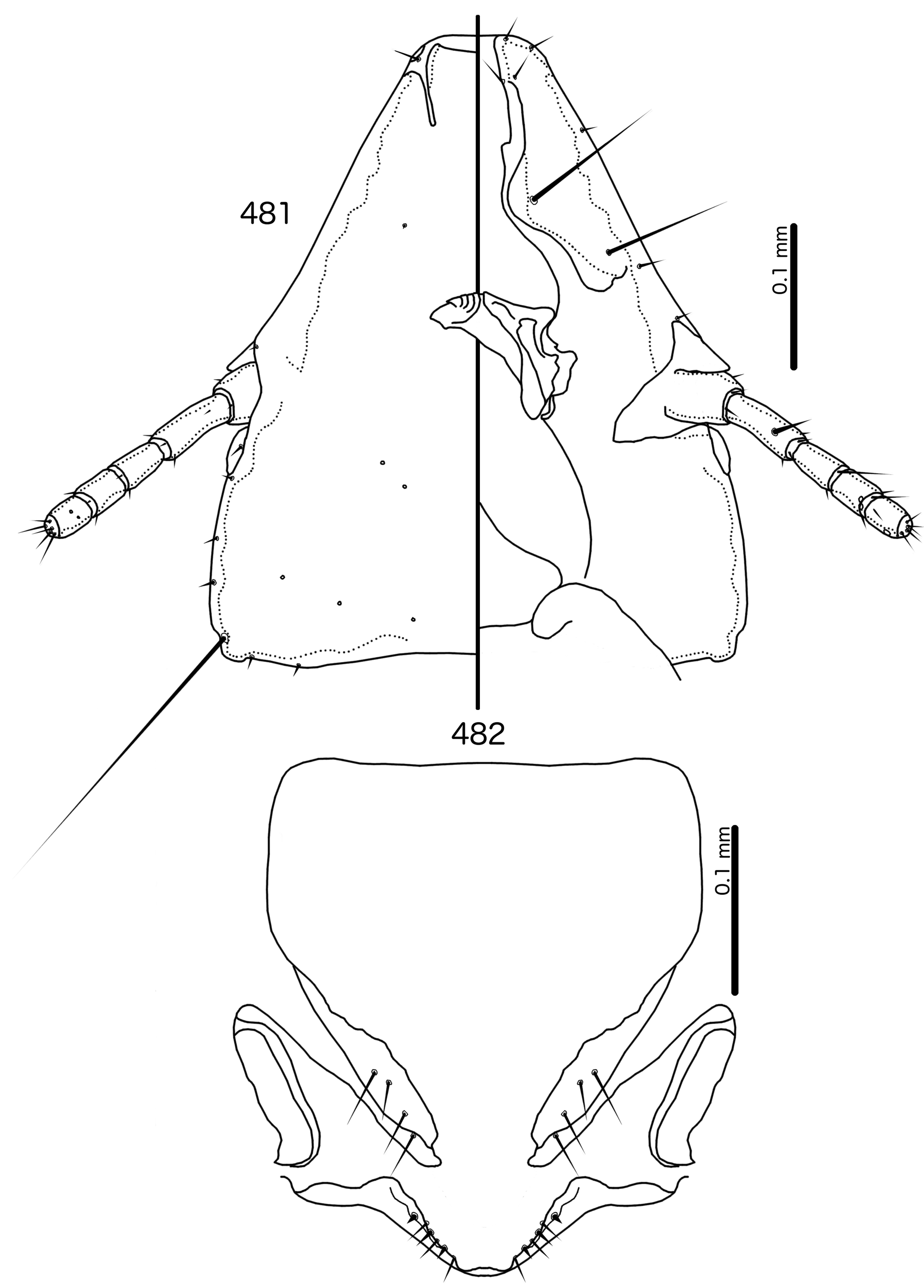
Diagnosis. Meropsiella is not particularly similar to any other genus treated here. Meropsiella was placed near Meropoecus and Motmotnirmus in the phylogeny of Bush et al. (2016), but this placement was not strongly supported. Morphologically, there are great differences among these three genera in many character sets; indeed, the male genitalia of Meropsiella and Meropoecus are so different that they are hard to compare. The marginal carina is interrupted submedianly in all three genera. However, the carina of Motmotnirmus is interrupted only submedianly ( Fig. 504 View FIGURES 504 – 508 ), whereas the carina of Meropoecus is reduced to a small remnant near the preantennal nodi ( Figs 494 View FIGURES 494 – 496 , 499 View FIGURES 499 – 501 ) and the marginal carina of Meropsiella is interrupted submedianly and laterally, at least on the dorsal side ( Fig. 481 View FIGURES 481 – 482 ). In Motmotnirmus ( Fig. 504 View FIGURES 504 – 508 ) and Meropsiella ( Fig. 481 View FIGURES 481 – 482 ) the dorsal preantennal suture reaches the dsms but not the ads, but in Meropoecus the suture reaches the ads as well ( Figs 494 View FIGURES 494 – 496 , 499 View FIGURES 499 – 501 ). The mts 2 are macrosetae in Motmotnirmus ( Fig. 504 View FIGURES 504 – 508 ), and mts 2 is longer than mts 1 and mts 4–5 in Meropoecus ( Figs 494 View FIGURES 494 – 496 , 499 View FIGURES 499 – 501 ), but in Meropsiella all mts except mts 3 are of equal length ( Fig. 481 View FIGURES 481 – 482 ). Gonopore is terminal in Motmotnirmus ( Fig. 506 View FIGURES 504 – 508 ), but ventral in Meropsiella ( Figs 484, 487, 490 View FIGURES 483 – 491 ), and other characters of the male genitalia are hard to compare between these three genera; the male genitalia of Meropoecus ( Figs 495 View FIGURES 494 – 496 , 500 View FIGURES 499 – 501 ) are unique within the Brueelia - complex, and most of the terminology introduced here to describe the male genitalia of the Brueelia -complex are largely inapplicable to Meropoecus . The female subgenital plate and vulval margin of Meropsiella ( Fig. 482 View FIGURES 481 – 482 ) is structurally very similar to that of Meropoecus ( Figs 496 View FIGURES 494 – 496 , 501 View FIGURES 499 – 501 ): both genera have broad lateral submarginal extensions of the subgenital plate, and in both genera, there is a distinct median extension of the subgenital plate, that may approach, but not reach, the vulval margin. Additionally, in both Meropoecus ( Figs 496 View FIGURES 494 – 496 , 500 View FIGURES 499 – 501 ) and Meropsiella ( Fig. 482 View FIGURES 481 – 482 ), the vss and vms are largely mixed, and do not form separate sets. All these characters are in stark contrast to Motmornirmus ( Fig. 508 View FIGURES 504 – 508 ), in which the subgenital plate does reach the vulval margin, but does not have lateral submarginal extensions, and in which the vss and vms are separated into distinct sets.
Description. Both sexes. Head trapezoidal ( Fig. 481 View FIGURES 481 – 482 ). Marginal carina interrupted submedianly, but not completely interrupted laterally. Ventral carina continuous with marginal carina. Frons hyaline and continuous with dorsal preantennal suture that does not reach ads. Dorsal anterior plate continuous with main head plate. Ventral anterior plate absent. Head setae as in Fig. 481 View FIGURES 481 – 482 ; ads very short, and may be absent in some species; mds and pns absent. Antennae monomorphic. Temporal carinae not visible; mts 3 only macrosetae. Temporal margin bulges distinctly between mts 3 and mts 4. Gular plate roughly triangular.
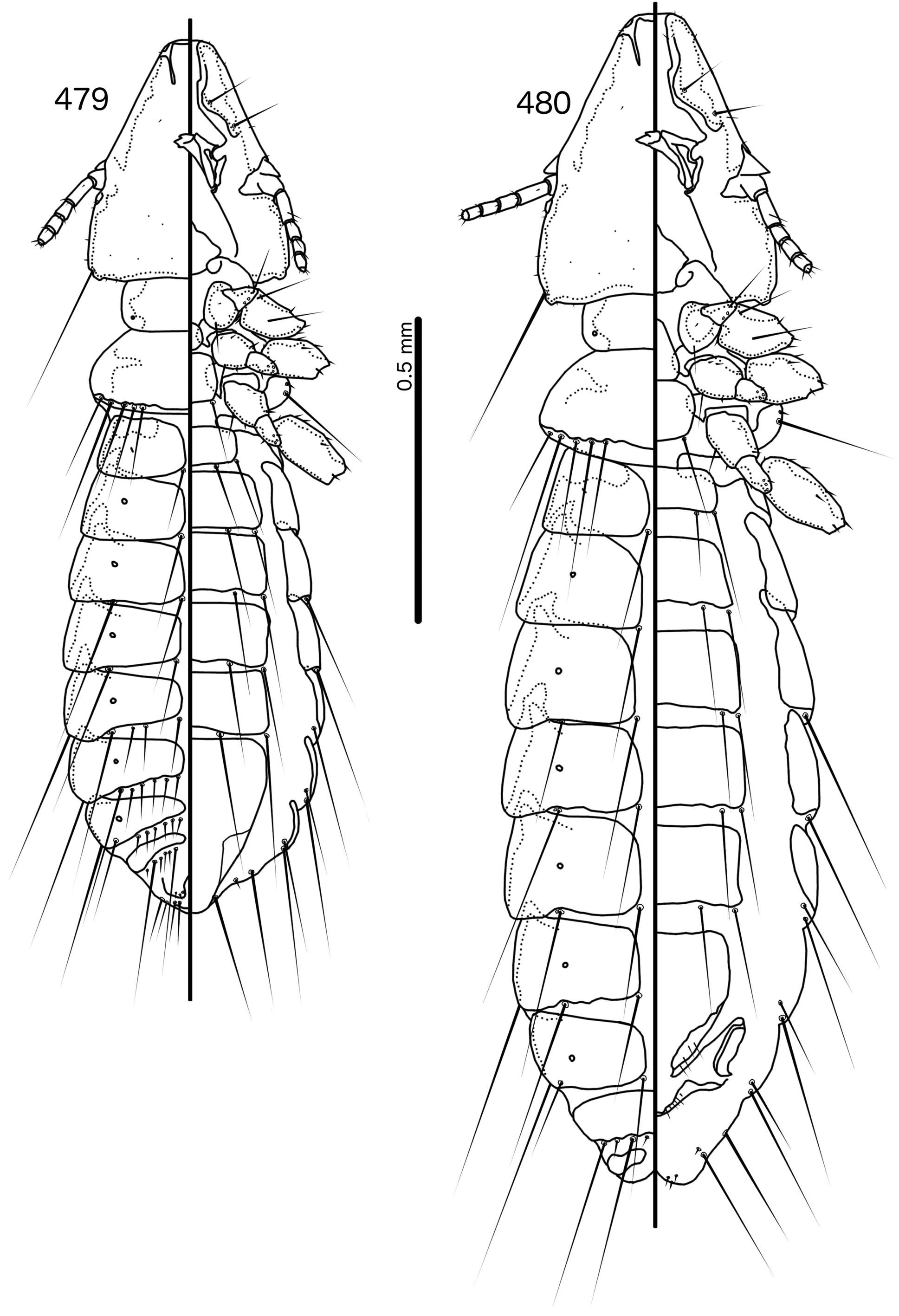
Prothorax ( Figs 479–480 View FIGURES 479 – 480 ) rounded rectangular; ppss absent or minute. Proepimera with hammer- or hookshaped median ends. Pterothorax rounded trapezoidal; lateral margins convex; posterior margin more or less flat ( contra Denny, 1842: 133 ); mms moderately separated medianly. Meso- and metasterna not fused, setae only on postero-lateral corners of metasternum. Metepisterna with hammer-shaped median ends. Leg chaetotaxy as in Fig. 25 View FIGURES 25 , except fI-v4, fI-p2, fII-v2, fIII-v2 absent; tII-v1 and tIII-v1 short.
Abdomen ( Figs 479–480 View FIGURES 479 – 480 ) elongated oval in both sexes, longer in female. Tergopleurites rectangular; tergopleurites II–IX+X in male and tergopleurites II–VIII in female moderately divided medianly. Sternal plates rectangular, medianly continuous, do not approach pleurites. Pleural incrassations moderate. Ventral section of tergopleurites moderate to wide. Re-entrant heads prominent. Anterior end of pleurite II with rectangular extensions medianly. Male subgenital plate rounded triangular, reaching posterior margin of abdomen. Female subgenital plate roughly triangular, approaching but not reaching vulval margin ( Fig. 482 View FIGURES 481 – 482 ). Lateral submarginal extensions present. Abdominal chaetotaxy as in Table 2. Vulval margin with median bulge ( Fig. 482 View FIGURES 481 – 482 ), vss and vms not forming separate rows; vos follow lateral margins of subgenital plate, not approaching vulval margin.
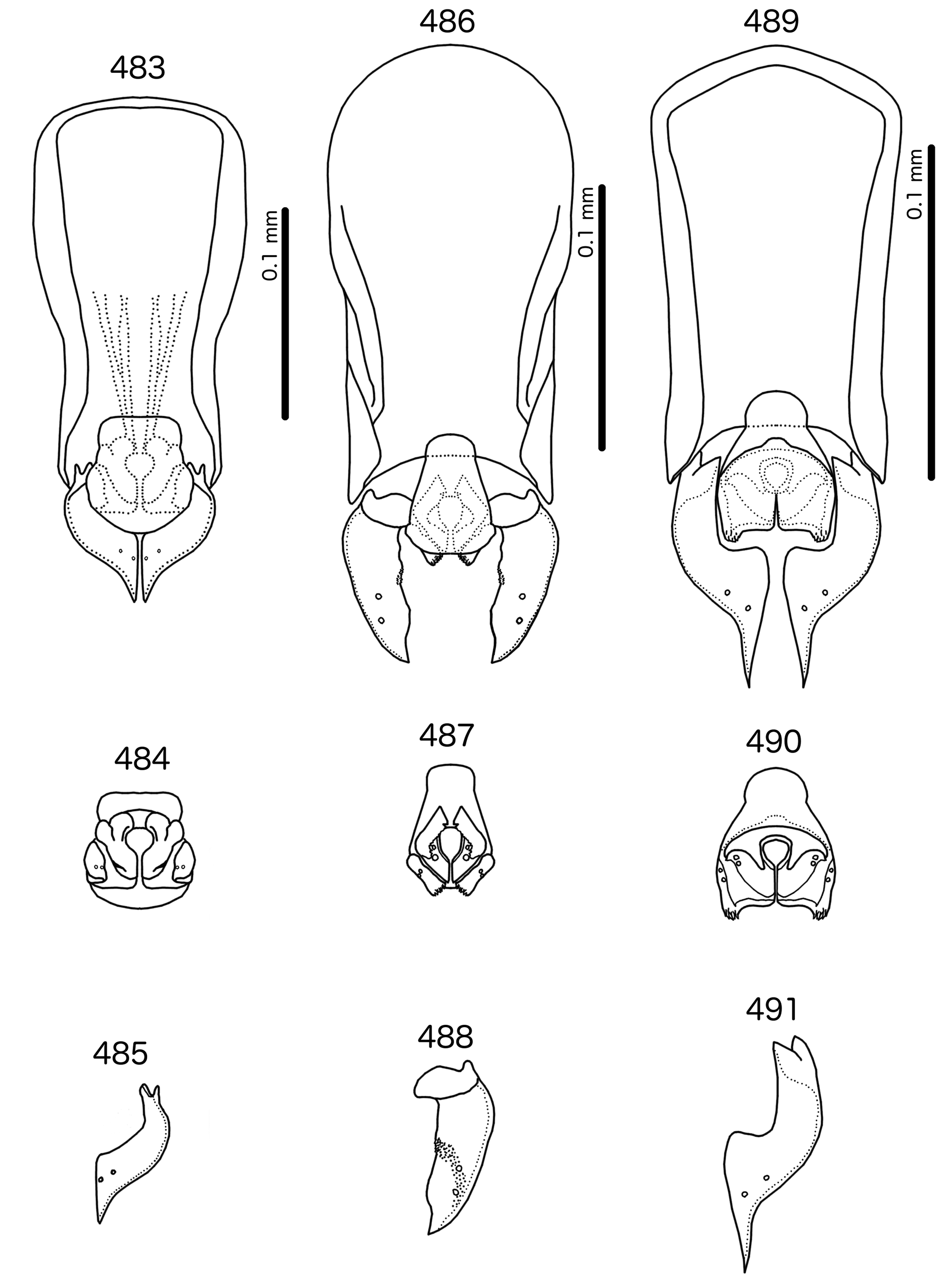
Male genitalia ( Fig. 483–491 View FIGURES 483 – 491 ) variable. Basal apodeme trapezoidal ( Fig. 483 View FIGURES 483 – 491 ), rounded rectangular ( Fig. 486 View FIGURES 483 – 491 ), or pentagonal ( Fig. 489 View FIGURES 483 – 491 ). Proximal mesosome blunt, either broad (e.g. Meropsiella apiastri , Fig. 484 View FIGURES 483 – 491 ) or narrow (e.g. Ms. bullockoda , Fig. 487 View FIGURES 483 – 491 ). Proximal mesosome overlapping basal apodeme. Gonopore with broad marginal thickening that vary in shape ( Figs 484, 487, 490 View FIGURES 483 – 491 ); open only distally. Mesosomal lobes fused distally, often square-shaped, but variable between species; 2 ames sensilla on each side lateral to gonopore; absent or not visible in Ms. apiastri ( Fig. 484 View FIGURES 483 – 491 ); 2 pmes sensilla on each side lateral or sublateral. Rugose nodi may be present as fringed or papillate sections of distal margins of mesosome ( Figs 487, 490 View FIGURES 483 – 491 ). Parameral heads bifid, but variable ( Figs 485, 488, 491 View FIGURES 483 – 491 ). Parameral blades generally broad, somewhat elongated, but those of Ms. bullockoda ( Fig. 488 View FIGURES 483 – 491 ) broad with irregular margins; pst1–2 both sensilla, central, not near distal end of parameres.
Host distribution. Species of this genus are only known from the genus Merops Linnaeus, 1758 , family Meropidae .
Geographic range. Occurs throughout Africa and Europe to South-East Asia and Australia.
Remarks. Meropsiella was erected by Conci (1941b: 104) for the slender lice parasitising Meropidae . Conci (1941b) stated that Meropsiella is similar to Brueelia , but distinguished by the almost perfectly triangular head shape, the distinct clypeal suture located at about 4/5ths of the length of the head, the presence of a long and a short seta on the frons, and the lack of setae on the posterior dorsal corners of the prothorax. He included only one species in the genus , Ms. apiastri , but suspected that Ms. erythropteri , which he was unable to examine, would be included as well. Hopkins & Clay (1952: 225) considered Meropsiella inseparable from Brueelia , a view followed also by Price et al. (2003: 198). In the phylogeny of Bush et al. (2016), Meropsiella was placed with strong support as a lineage sister to the main Brueelia -complex, together with several other genera ( Buerelius, Couala , Meropoecus , and Motmotnirmus ) that parasitise non-passerines. Mey & Barker (2014) suggested that Meropsiella should be resurrected, but offered no reasons for their opinion.
To date, the only revision of the Brueelia -complex species on the bee-eaters is by Williams (1981), who treated five species, three of which were newly described in that paper. However, we recognise Brueelia bullockoda Williams, 1981 as the only species belonging to Meropsiella , because we place Br. athertona Williams, 1981 in Aporisticeras n. gen., and Brueelia superciliosa Williams, 1981 is a junior synonym of Alcedoffula alcedinis ( Denny, 1842: 48) (see Williams 1982b). We have examined a large amount of lice from several species of beeeaters and found small but consistent differences between material we list as Ms. erythropteri in Appendix III; populations of Ms. erythropteri on different host species may be separate species.
Included species
* Meropsiella apiastri ( Denny, 1842: 52) [in Nirmus ] * Meropsiella bullockoda ( Williams, 1981: 516) [in Brueelia ] new combination * Meropsiella erythropteri ( Piaget, 1885: 28) [in Nirmus ]
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Meropsiella Conci, 1941
| Bush, Sarah E. 2017 |
Degeeriella
| Keler 1936: 257 |
| Neumann 1906: 60 |
Meropsiella apiastri ( Denny, 1842: 52 )
| Williams 1981: 516 |
| Piaget 1885: 28 |
| Denny 1842: 52 |
Nirmus
| Nitzsch 1818: 291 |