Saepocephalum Gustafsson & Bush, 2017
|
publication ID |
https://doi.org/10.11646/zootaxa.4313.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A5Fdfba5-F992-44A8-84C2-1756C943C19B |
|
DOI |
https://doi.org/10.5281/zenodo.5296957 |
|
persistent identifier |
https://treatment.plazi.org/id/832187E9-FF1F-FF55-FF74-6298FDBFFC36 |
|
treatment provided by |
Plazi |
|
scientific name |
Saepocephalum Gustafsson & Bush |
| status |
gen. nov. |
Saepocephalum Gustafsson & Bush , new genus
Type species. Saepocephalum stephenfryi new species
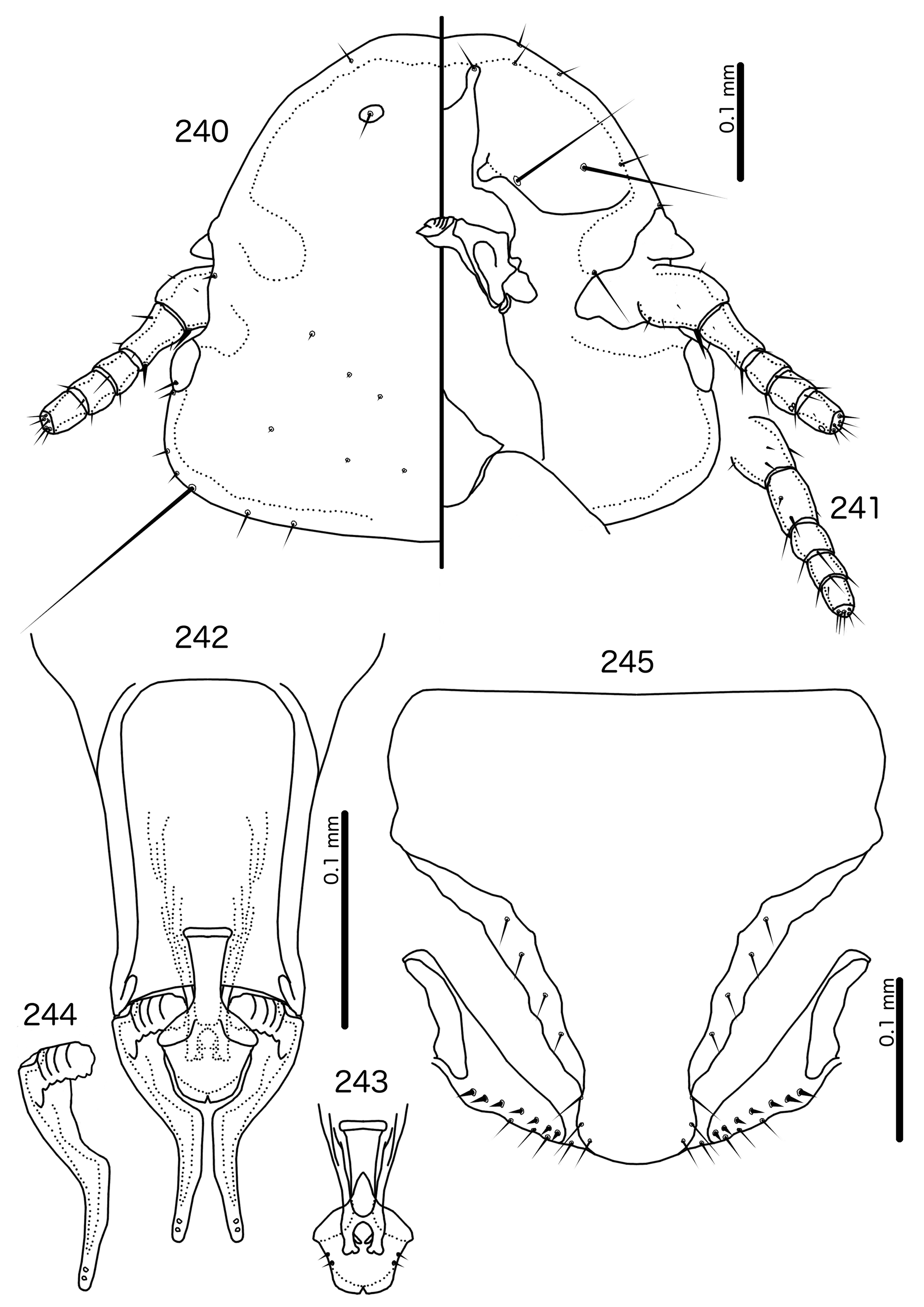
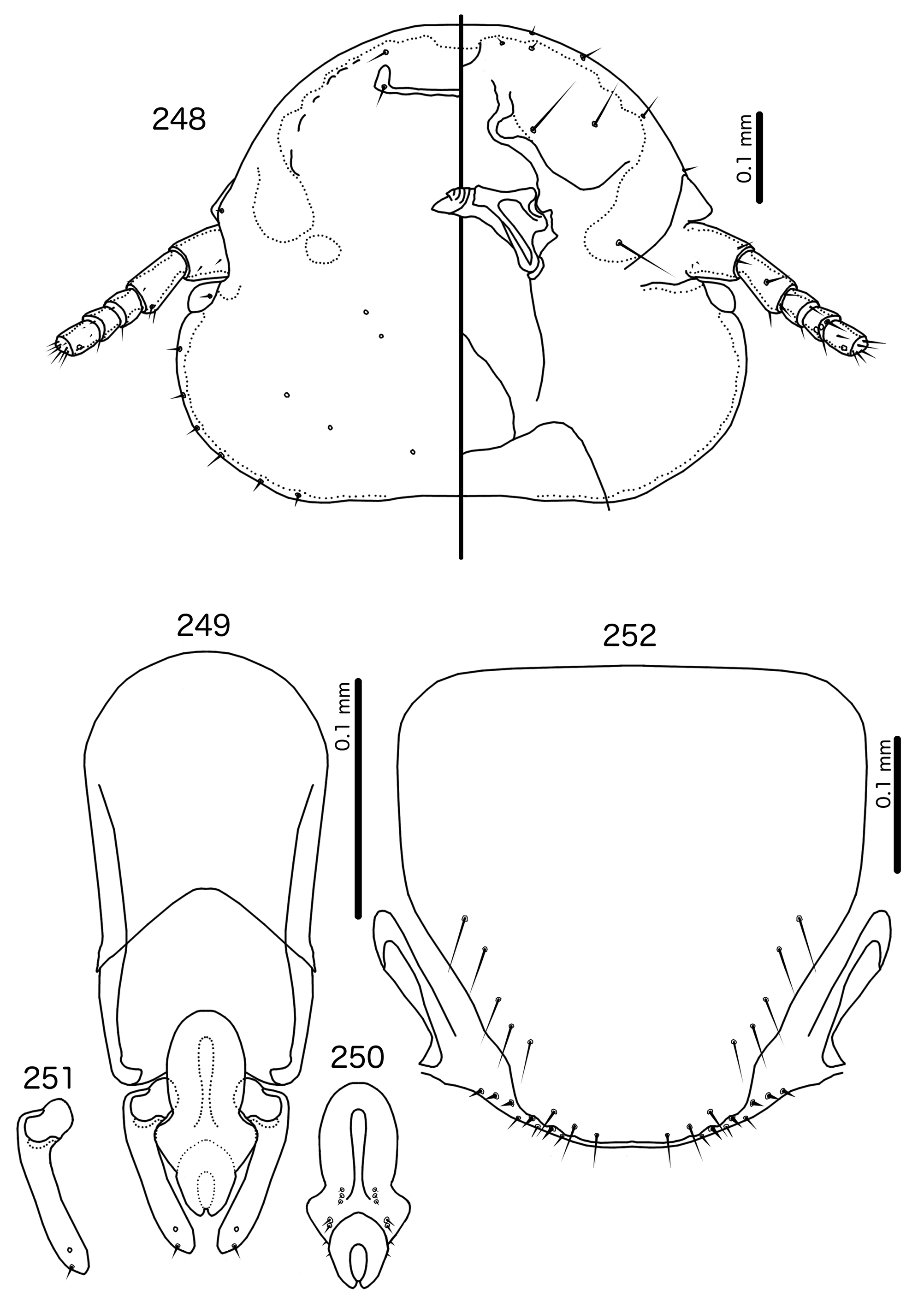
Diagnosis. Saepocephalum n. gen. does not appear to be morphologically close to any other genus treated here. The fused ventral anterior plate, undisplaced marginal carina ( Fig. 240 View FIGURES 240 – 245 ), and clypeo-labral suture that does not reach the anterior margin of the head is shared by only two other genera in the Brueelia -complex: Harpactrox n. gen. ( Figs 248 View FIGURES 248 – 252 , 255, 260) and Anarchonirmus n. gen. ( Fig. 116 View FIGURES 116 – 121 ). Other than these characters, neither of these genera appear to be close to Saepocephalum .
Both Anarchonirmus ( Figs 116–117 View FIGURES 116 – 121 ) and Saepocephalum n. gen ( Figs 240–241 View FIGURES 240 – 245 ) have sexually dimorphic antennae, and as3 is absent in both genera ( Figs 116 View FIGURES 116 – 121 , 240 View FIGURES 240 – 245 ). Both genera also lack dorsal preantennal sutures, apart from the isolated suture around the ads in Saepocephalum ( Fig. 240 View FIGURES 240 – 245 ). In both Anarchonirmus ( Figs 114–115 View FIGURES 114 – 115 ) and Saepocephalum ( Figs 238–239 View FIGURES 238 – 239 ) the tergopleurites do not reach the lateral margins of the abdomen. However, abdominal chaetotaxy is very different in these two genera ( Table 2), and the modifications of the male flagellomeres seen in Anarchonirmus ( Fig. 116 View FIGURES 116 – 121 ) are not seen in Saepocephalum ( Fig. 240 View FIGURES 240 – 245 ). In both genera there is sclerotization of the vulval margin, but in Anarchonirmus ( Fig. 121 View FIGURES 116 – 121 ) this cross-piece medianly displaced, but complete, whereas in Saepocephalum ( Fig. 245 View FIGURES 240 – 245 ) these sclerotized plates are laterally detached. The proximal mesosome of Anarchonirmus ( Fig. 118 View FIGURES 116 – 121 ) does not overlap with the basal apodeme, and is not thickened anteriorly; in Saepocephalum ( Fig. 242 View FIGURES 240 – 245 ) the mesosome overlaps with the basal apodeme and is thickened anteriorly. Gonopore is terminal in Anarchonirmus ( Fig. 119 View FIGURES 116 – 121 ), but ventral in Saepocephalum ( Fig. 243 View FIGURES 240 – 245 ), and the mesosomal lobes are fused distally in Saepocephalum , but separate distally in Anarchonirmus . Parameral heads are blunt and simple in Anarchonirmus ( Fig. 120 View FIGURES 116 – 121 ), but folded medianly and with complex ornamentations in Saepocephalum ( Fig. 244 View FIGURES 240 – 245 ).
Similarities between Saepocephalum and Harpactrox are slightly larger, as in both genera as3 are absent ( Figs 240 View FIGURES 240 – 245 , 248 View FIGURES 248 – 252 ), parameral heads are folded medianly ( Figs 244 View FIGURES 240 – 245 , 251 View FIGURES 248 – 252 ), and proximal mesosome overlaps with basal apodeme ( Figs 242 View FIGURES 240 – 245 , 249 View FIGURES 248 – 252 ). In addition, in both Harpactrox ( Figs 246–247 View FIGURES 246 – 247 ) and Saepocephalum ( Fig. 238–239 View FIGURES 238 – 239 ) psps are absent on female tergopleurites II–III and male tergopleurite II; these are present in in Anarchonirmus ( Figs 114–115 View FIGURES 114 – 115 ). A dorsal preantennal suture is typically present in both Saepocephalum ( Fig. 240 View FIGURES 240 – 245 ) and Harpactrox ( Figs 248 View FIGURES 248 – 252 , 255, 260), this suture is medianly continuous in Harpactrox , but, when present, isolated around the ads in Saepocephalum . All mts are microsetae in Harpactrox ( Figs 248 View FIGURES 248 – 252 , 255, 260), but mts 3 is a macroseta in Saepocephalum ( Fig. 240 View FIGURES 240 – 245 ). The subgenital plate does not form a cross-piece in Harpactrox ( Figs 252 View FIGURES 248 – 252 , 259, 262), unlike in Saepocephalum ( Fig. 245 View FIGURES 240 – 245 ) which has a laterally detached cross-piece. Parameral heads are folded medianly in both genera, but those of Harpactrox ( Figs 251 View FIGURES 248 – 252 , 258) are much simpler than those of Saepocephalum ( Fig. 244 View FIGURES 240 – 245 ), and the parameral blades are more elongated and angular in Saepocephalum than in Harpactrox . pst1–2 are both sensilla in Saepocephalum ( Fig. 244 View FIGURES 240 – 245 ), but pst2 are microsetae in Harpactrox ( Figs 251 View FIGURES 248 – 252 , 258). Gonopore is terminal in Harpactrox ( Figs 250 View FIGURES 248 – 252 , 257), but ventral in Saepocephalum ( Fig. 243 View FIGURES 240 – 245 ). No ames are visible in Saepocephalum ; these are microsetae in Harpactrox ( Figs 250 View FIGURES 248 – 252 , 257).
Description. Both sexes. Head broad, convex-dome shaped ( Fig. 240 View FIGURES 240 – 245 ). Marginal carina uninterrupted. Clypeolabral suture does not reach anterior margin of head. Dorsal preantennal suture present only around apertures of ads in some specimens. Ventral carinae diffuse anterior to pulvinus and not clearly continuous with marginal carina. Ventral anterior plate present, continuous with marginal carina. Head setae as in Fig. 240 View FIGURES 240 – 245 ; as3 absent. Coni small. Antennae sexually dimorphic, with male scapes ( Fig. 240 View FIGURES 240 – 245 ) about twice the length of that of female ( Fig. 241 View FIGURES 240 – 245 ), and slightly swollen. Temporal carinae not visible; mts 3 only macrosetae. Gular plate spade-shaped.
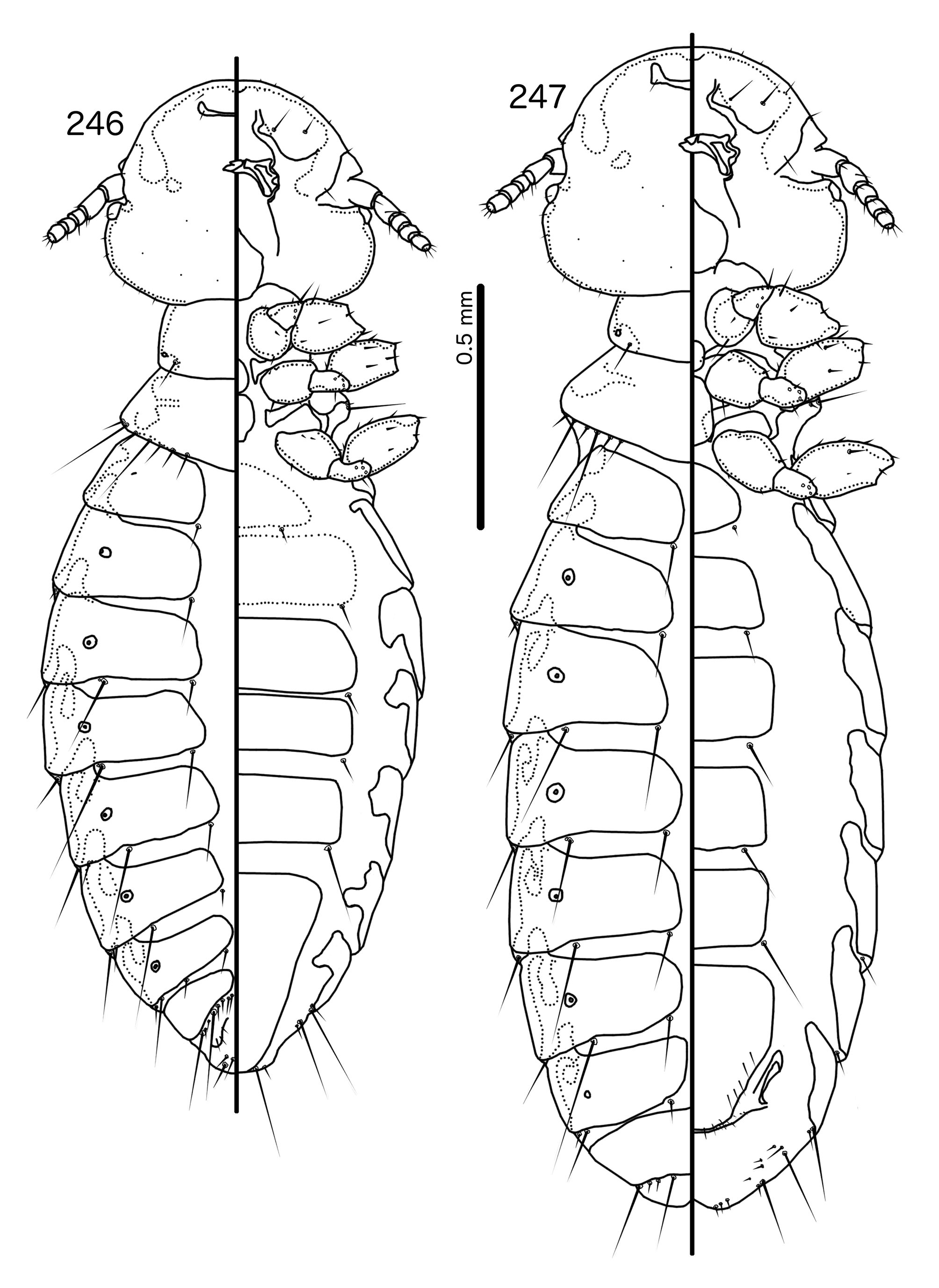
Prothorax small, rectangular ( Figs 238–239 View FIGURES 238 – 239 ); ppss on postero-lateral corner. Proepimera slender, hook-shaped, curling around coxae II. Pterothorax trapezoidal to pentagonal; lateral margins divergent; posterior margin either roughly flat or vaguely convergent to median point. Meso- and metasterna not fused; setae only on postero-lateral corners of mesosternum; metasternum nude. Metepisterna broad, median ends blunt. mms moderately interrupted medianly. Leg chaetotaxy as in Fig. 25 View FIGURES 25 , except fI-p2, fII-v2, fIII-v2 absent; fI-v4 absent in males, but present, spikelike in females.
Abdomen ( Figs 238–239 View FIGURES 238 – 239 ) oblong in female, more oval in male. Abdominal chaetotaxy as in Table 2 and Figs 238–239 View FIGURES 238 – 239 . Tergopleurites rectangular; tergopleurites II–IX+X in male and tergopleurites II–VIII in female narrowly separated medianly. Tergopleurites do not reach lateral margins of abdomen. Sternal plates rectangular, not approaching lateral margins of abdomen. Pleural incrassations of segments II–VIII in both sexes as latitudinal thickenings on antero-lateral margin of tergopleurites. Male subgenital plate roughly triangular, with sinuous lateral margin, reaching posterior margin of abdomen. Female subgenital plate roughly pentagonal, distal lateral margins concave. Lateral marginal plates present, detached from subgenital plate ( Fig. 245 View FIGURES 240 – 245 ). Vulval margin ( Fig. 245 View FIGURES 240 – 245 ) with slender vms, thorn-like vss; vos follow lateral margins of subgenital plate; distal vos median to vss.
Male genitalia ( Figs 242–244 View FIGURES 240 – 245 ) distinct. Basal apodeme roughly rectangular, anterior margin diffuse, flaring. Proximal mesosome slender, elongated, overlapping with basal apodeme, and with proximal margin thickened. Gonopore ( Fig. 243 View FIGURES 240 – 245 ) ventral, open distally, continuous with subparallel ventral ridges. Mesosomal lobes rounded, fused distal to gonopore; 2 pmes microsetae visible on lateral margin of lobes on either side. Parameral heads ( Fig. 244 View FIGURES 240 – 245 ) folded, rectangular, with serrated posterior margins and several ridges on dorsal side. Parameral blades slender and elongated; pst1–2 sensilla, central, near distal tip of paramere.
Host distribution. Saepocephalum is monotypic, restricted to Corcorax melanoramphos (Vieillot, 1817) . No lice of the Brueelia -complex are known from the only other species in the family Corcoracidae : Struthidea cinerea Gould, 1837 .
Geographical range. Australia.
Etymology. The genus name is derived from the Latin “ saepes ” for “fence”, and Greek “ kefali ”, meaning “head”. This refers to the dorsally and ventrally complete marginal carina ( Fig. 240 View FIGURES 240 – 245 ), a condition that is rare within the Brueelia -complex. Gender: neuter.
Remarks. In the phylogeny of Bush et al. (2016), Saepocephalum was placed inside a clade containing Traihoriella and Bizarrifrons , neither of which resembles Saepocephalum morphologically. The support values for all deeper association in this clade were low, and the association of Saepocephalum with Traihoriella and Bizarrifrons is not supported.
Included species
* Saepocephalum stephenfryi new species
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |