Dichotomaria huismanii C.W. Schneid., Popolizio et Spagnuolo, 2016
|
publication ID |
https://doi.org/ 10.1515/bot-2015-0068 |
|
persistent identifier |
https://treatment.plazi.org/id/83772245-D450-D246-FF53-FBB0F5F694EC |
|
treatment provided by |
Felipe |
|
scientific name |
Dichotomaria huismanii C.W. Schneid., Popolizio et Spagnuolo |
| status |
sp. nov. |
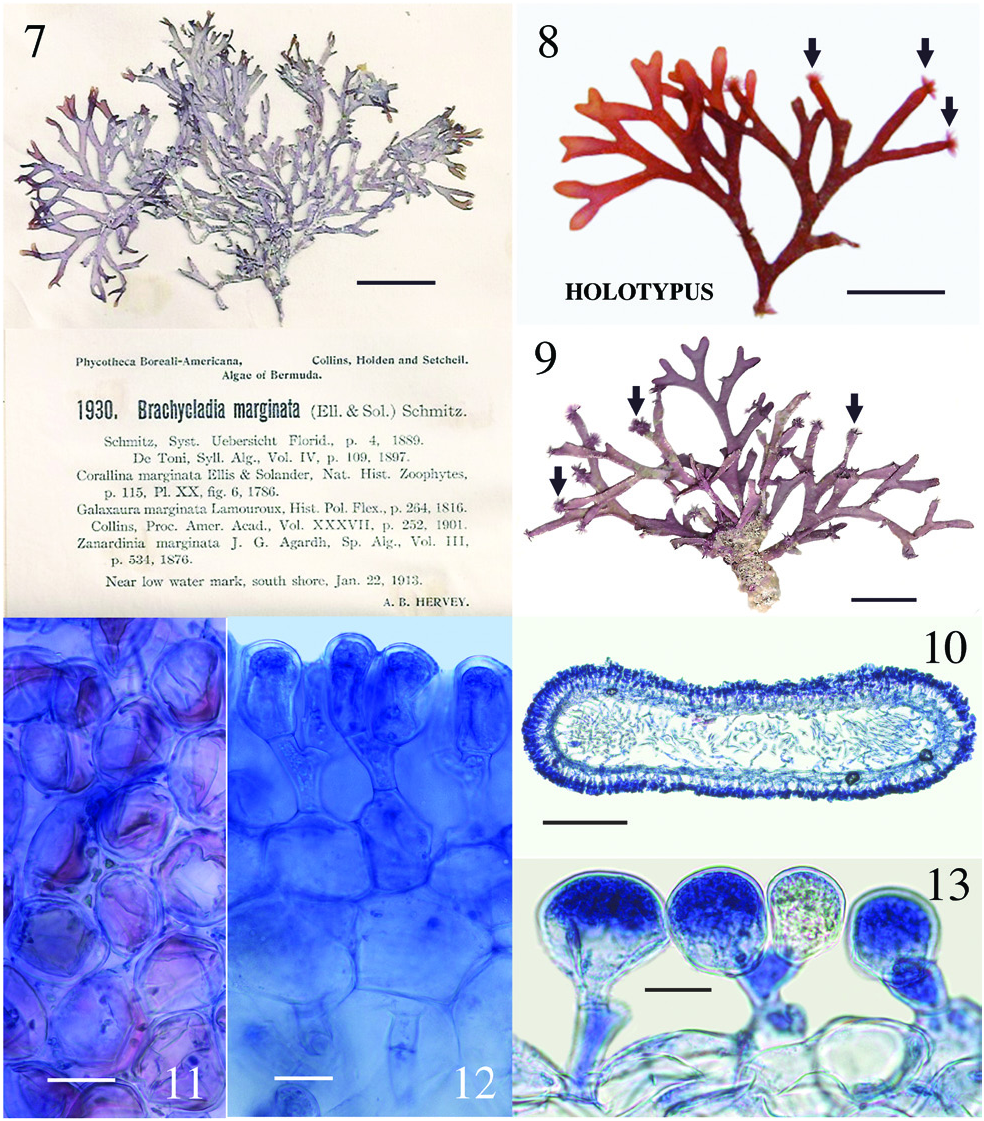
Dichotomaria huismanii C.W. Schneid., Popolizio et Spagnuolo sp. nov. ( Figures 7–13 View Figures 7–13 )
Description
Rosy-brown plants to 8 cm tall and 12 cm across, composed of dichotomously branched, flattened axes with thickened margins above, the axes subterete below; axes mottled in appearance when dried, annulations, when present, faint; internodes 3–9 mm long and 1.2–2.0 mm wide; axes branching dichotomously at angles of (32–) 41–54 (–68°); cortex of sporophytes composed of two layers of cells, outer cortical assimilatory cells subspherical to ovoid, 28–50 µm length and 22–48 µm diameter, and an inner layer of flared stalk cells, each bearing one or two assimilatory cells, 23–45 µm length and 5–13 µm diameter at narrowest point; stalk cells borne typically in pairs though occasionally alone on the outer layer of the inner cortex; stalk cells unicellular; inner cortex consisting of two layers of transversely ovoid to rectangular cells, 24–44 (–70) µm long and (38–) 58–89 µm diameter; subcortical cells subspherical at margins; medullary filaments extending from subcortical cells, closely adherent and parallel, running longitudinally the length of the axis, 13–20 µm diameter; tetrasporangia and gametophytes unknown.
Etymology
The epithet “ huismanii ” honors Dr. John M. Huisman, the foremost expert on the Nemaliales today, for, among other things, his prolific systematic work on the order over the past three decades, culminating in his recognition of the D. marginata complex using genetic techniques ( Huisman et al. 2004, Kurihara and Huisman 2006).
Holotype
T. R. Popolizio 12-117-5 [ BDA1492 ], 20 Sept.2012, Hog Breaker , north shore Bermuda I., 32°27′47.7″N, 64°49′48.9″W, Bermuda, western Atlantic Ocean, depth 12 m [ MICH] ( Figure 8 View Figures 7–13 ); isotype Herb. CWS. GoogleMaps
Paratypes
Bermuda – A. B. Hervey , P. B.- A. no. 1930 [ Collins et al. 1913, as Brachycladia marginata ], 22 Jan. 1913, near low water mark south shore , Bermuda I. [Herb. CWS] ( Figure 7 View Figures 7–13 ); CWS / CEL 10-5 View Materials - 13 View Materials [ BDA0024 ], 19 August 2010, reef off Frick’s Beach, 32°19′56.0″N, 64°40′20.7″W, Tucker’s Town GoogleMaps , Bermuda I., depth 10–12 m ( Figure 9 View Figures 7–13 ); TRP / CWS 12-151 - 11 [ BDA1669 ], TRP / CWS 12-158 - 3 [ BDA1709 ], TRP / CWS 12-170 - 9 [ BDA1805 ] (see Table 1 for collection details) .
Distribution
Endemic to Bermuda as presently known.
Remarks
The new species is presently very rare in Bermuda and, when found, there are few individuals in the population. When Collins and Hervey (1917) first reported Galaxaura marginata from Bermuda, they cited only their shallow water collection from Gravelly Bay ( P. B.- A. no. 1930; Figure 7 View Figures 7–13 ), but clearly there were enough specimens available at the time to make herbarium specimens for each of the 80 distributed sets of the exsiccata they were ultimately placed in ( Fahey and Doty 1955). Collins and Hervey (1917) did not distinguish their collections in any feature from the standard characteristics of G. marginata taken from earlier sources available to them at the time. Interestingly, all of the Dichotomaria huismanii specimens we have collected show many truncated branch tips that have been grazed by herbivores ( Figures 8 and 9 View Figures 7–13 ). Often on these plants, closely adherent medullary filaments that run longitudinally through the axes emerge from the truncated tips, and occasionally from branch nodes, as caespitose tufts of loose, uniseriate filaments, appearing much like a dense epiphytic growth of an acrochaetioid alga ( Figures 8 and 9 View Figures 7–13 ; we sequenced these tufts and they were a 100% match to the macroscopic plant on which they form). Taylor (1960) may have observed the same for D. marginata when he mentioned “branches occasionally tipped with a brush of deciduous hairs”, but he made no mention of herbivory. It is worth noting that all of our recent collections from Bermuda are much smaller (to 4 cm tall, 7 cm across) and less robust than those distributed in 1913 fascicles of P. B.- A (8 cm tall, 12 cm across; Figure 7 View Figures 7–13 ). The reduced habit appears to be a trend seen in many seaweeds collected in Bermuda during the latter part of the 20th and first part of the 21st centuries, these being smaller than herbarium collections made in the early years of the 20th century. We believe increased fish herbivory in Bermuda may be responsible for the more diminutive plants of today, as was shown for two small Botryocladia spp. growing on the reefs compared to large, fully formed, non-grazed plants in display tanks of the Bermuda Aquarium ( Schneider and Lane 2008). Increased herbivory could also account for the fewer individuals found today in Bermuda populations as compared with the distant past, as well as a lack of specimens from shallow waters, the only habitat reported for this species by Collins and Hervey (1917).
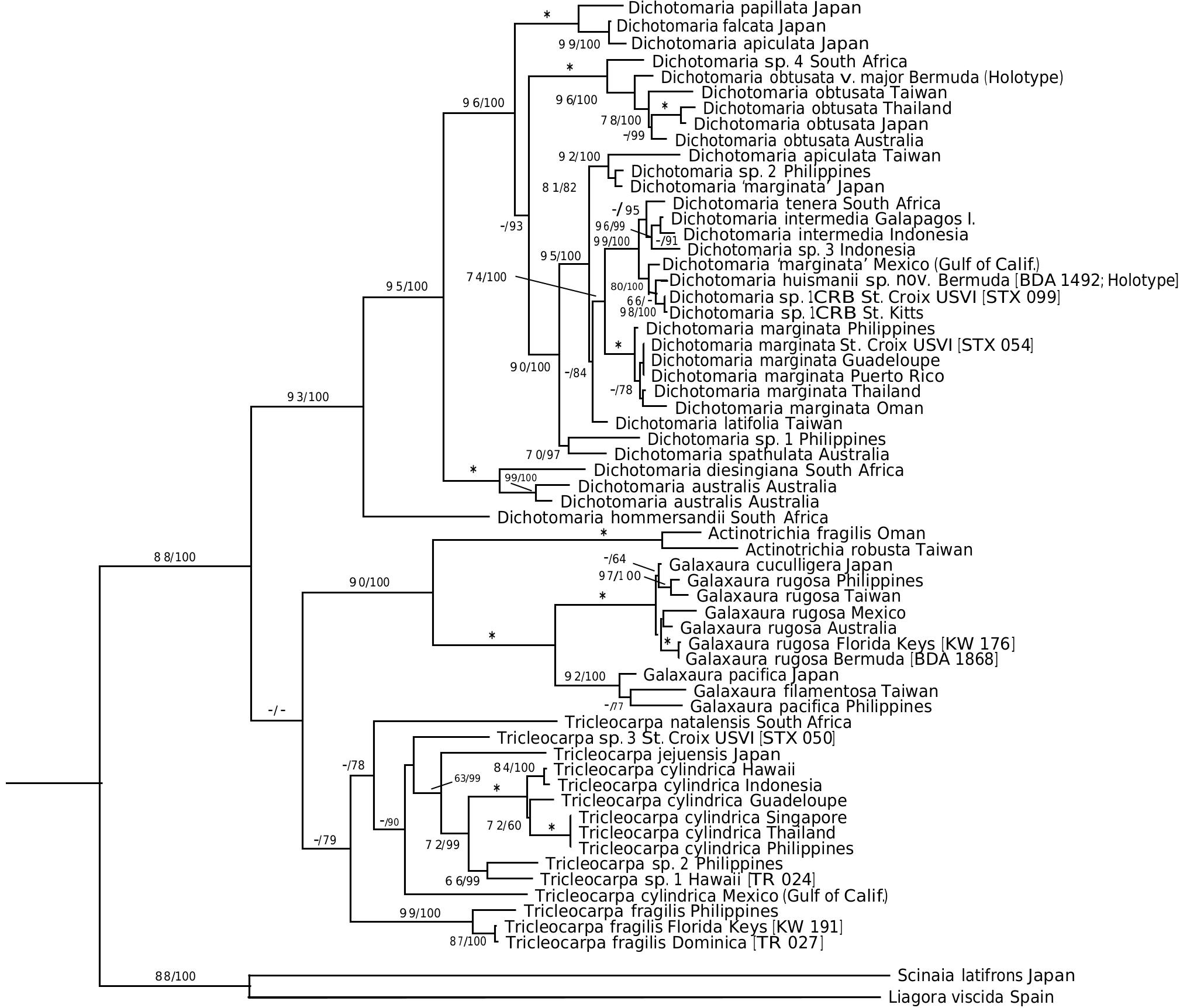
Thus far, the new species is known only from Bermuda and represents the only flattened member of the genus in the local flora. Dichotomaria huismanii is genetically distinct from D. marginata in the West Indies (4.8% bp differences in COI-5 P sequences, 2.4% in rbc L), the islands of Bermuda being isolated from the closest populations of the generitype by a distance> 1350 km to the northeast of the Bahamas. St. Kitts /St. Croix isolates ( Dichotomaria sp. 1 CRB, Table 1) are sister to the new species in the COI-5 P tree ( Figure 1 View Figure 1 ), but show 4.1% bp differences with it (22 nucleotide differences out of 538). The rbc L clade where D. huismanii is resolved ( Figure 2 View Figure 2 ) also includes a specimen identified as “ D. marginata ” from the Gulf of California (GenBank AY688022). The rbc L sequences from Bermuda and Mexico are 0.6% different from each other (there is no COI-5 P data for the Mexican isolate). The Bermuda and Mexico species are each 0.6% different in rbc L bp from the two Caribbean sequences of Dichotomaria sp. 1 CRB ( Table 1).
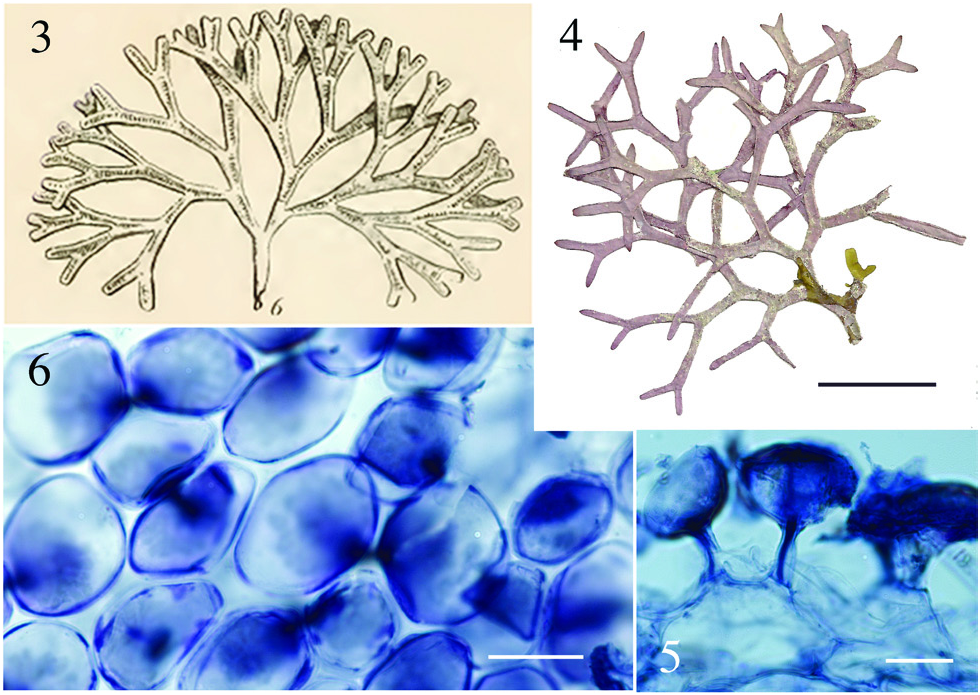
Aside from the geographic separation and genetic difference, a few morphological characteristics present themselves as means for differentiating D. huismanii from its cryptic counterpart in the western Atlantic, D. marginata . In gross morphology, D. huismanii is difficult to distinguish from D. marginata , but subtle differences can be found anatomically. The dimensions of cortical cells, thallus height and width, and the length and width of internodes all demonstrate a great amount of overlap between the two species ( Table 2). But unlike D. marginata , none of the assimilatory cells on D. huismanii are apiculate ( Figures 11–13 View Figures 7–13 ), a character that may be of use in western Atlantic populations of Dichotomaria after a more thorough examination of isolates is made from the region of the Bahamas, throughout the Caribbean and south to Brazil. The new species has four cortical cell layers including stalk and outer assimilatory cells ( Figure 12 View Figures 7–13 ) and in general has greater diameter medullary filaments (13–20 µm) than the generitype D. marginata (7–13 µm), which mostly shows three cortical cell layers ( Figure 5 View Figures 3–6 ). The dichotomous axes of D. huismanii branch at markedly narrower angles than St. Croix specimens of D. marginata , 32–68° vs. 72–89°. Whether any of these anatomical character differences will hold up as more isolates from the Caribbean are sequenced remains to be seen.
Using the chloroplast rbc L gene, along with the western Atlantic species mentioned above, D. huismanii clusters with two Indo-Pacific species, D. tenera and D. intermedia ( Figure 2 View Figure 2 ). The only detailed description of D. tenera since its genetic analysis and resurrection by Huisman et al. (2004) is found in De Clerck et al. (2005) based on specimens from Kwazulu-Natal, South Africa, the geographic area from which Huisman et al. (2004) used isolate sequences to segregate it from Caribbean D. marginata . These Indian Ocean specimens have somewhat broader flattened branches (1.5– 3.0 mm) than D. huismanii (1.2–2.2 mm), and unlike the new species have axes that are hirsute above the holdfasts and produce outer cortical cells that are apiculate at the margins ( De Clerck et al. 2005). Specimens of D. tenera from Mauritius are reported to have outer cortical cells that are 38–42 µm tall and 27–30 µm diameter ( BØrgesen 1942, as Galaxaura tenera Kjellman ), well within the range of these cell sizes in D. huismanii . Their branch angles (measured from BØrgesen 1942, figure 24) are from 50–75°, a slightly wider angle range than that of the new species reported here ( Table 2). A specimen attributed to D. tenera from South Africa and pictured by Kylin (1938), as G. tenera ), has much narrower branch angles, 40–55°, than the Mauritian sample.
The second species that genetically clusters with Dichotomaria huismanii ( Figure 2 View Figure 2 ), D. intermedia (R.C.Y. Chou) Wiriyadamrikul, M.J. Wynne et S.M. Boo (type locality= Galapagos Is.), is considerably larger (to 23 cm) than the new species (to 8 cm). Despite its overall large size, D. intermedia has similar cell dimensions for outer cortical cells on tetrasporophytes, 30–50 µm tall and 25–35 µm diameter ( Wiriyadamrikul et al. 2014), as compared to those for D. huismanii , 28–50 µm tall and 22–48 µm diameter ( Table 2), but unlike the new species, these cells are apiculate.
Morphological comparisons of Galaxaura and Dichotomaria species from locations other than their type localities with species from different parts of the world create similar problems to those we have already alluded to for genetic data from distant locations. For example, prior to the availability of molecular sequencing and comparison of species, Chou (1945) used Pacific Costa Rican samples of G. stupocaulon Kjellman (type locality= Brazil) to characterize this species, and make comparisons with other members that would be included in the D. marginata complex today ( Huisman et al. 2004, Wang et al. 2005, Kurihara and Huisman 2006, Wiriyadamrikul et al. 2014). Oliveira (1977) noted the “great similarity” of Galaxaura angustifrons Kjellman (type locality= Brazil) from Brazil to G. veprecula Kjellman (type locality= Madagascar) from the Philippines and Ecuador based on Chou’s (1947) observations and measurements. G. veprecula was subsequently placed in synonymy with G. tenera by Papenfuss and Chiang (1969), species later subsumed in G. marginata ( Papenfuss et al. 1982) . Finally, G. tenera was resurrected as Dichotomaria tenera by Huisman et al. (2004) based on LSU sequence data (and the morphology of terminal cortical cells) of specimens from South Africa. It seems obvious from these convoluted pathways that historical taxonomic decisions involving Galaxaura and Dichotomaria species need to be reassessed from the fresh perspective of DNA sequencing, as has been done in selected recent studies ( Huisman et al. 2004, Wang et al. 2005, Kurihara and Huisman 2006, Wiriyadamrikul et al. 2014). Accordingly, what has been combined in the past under D. marginata , has already begun fragmenting back to a greater number of species.
For their Indian Ocean and Australian specimens of Dichotomaria “marginata ”, Huisman et al. (2004) were able to resurrect species names for flattened taxa from those areas with specimens that did not genetically match the sequence of true D. marginata from Puerto Rico. The resurrected D. australis and D. tenera had been among a large number of species that, over the years and culminating with the Papenfuss et al. (1982) circumscription, were placed in synonymy with D. marginata . In the western Atlantic, there are early names presently considered junior synonyms of D. marginata : G. angustifrons , G. frutescens Kjellman (type locality= Brazil), G. occidentalis and G. stupocaulon . None of these taxa were ever reported from Bermuda ( Taylor 1960). The above three species presently in the D. marginata complex with type localities in Brazil do share some resemblance to both D. marginata and D. huismanii , and are presently considered synonyms of the former. Observations for these species on type specimens is critical to linking anatomical measurements/characteristics to recently collected genetic species from near their type localities in this highly cryptic complex of species. As none of the historical species were described locally, we choose to describe our Bermuda specimens as a new species from the northern limit of distribution of the genus in the western Atlantic. Genetic information from other areas to the south may prove this species to have a more widespread distribution than presently delimited.
The separation and description of a new cryptic species from Bermuda for records that in the past were considered a part of a pantropical species’ range with a western Atlantic type is similar to other nemalialean examples already genetically distinguished in the islands. What was identified in Bermuda as Helminthocladia calvadosii (J.V. Lamouroux ex Duby) Setchell for over a century was shown to be H. kempii Popolizio, Chengsupanimit et C.W. Schneider ( Popolizio et al. 2013) , and what was known as Liagora ceranoides J.V. Lamouroux since the early 1900s in the islands was recently described as L. nesophila Popolizio, C.W. Schneider et C.E. Lane ( Popolizio et al. 2015) .
Acknowledgments: We thank Dr. Nina Lundholm and the Natural History Museum of Denmark [ C] for a loan of the holotype of Galaxaura occidentalis, Dr. Michael Wynne [ MICH] for a loan of Caribbean specimens, and Eric Salomaki for helping collect in St. Croix and sequencing the holotype of the new species. Dr. Struan Smith of the Bermuda Natural History Museum and Chris Flook of St. David’s , Bermuda, kindly provided logistical support for several collecting trips in Bermuda. CWS and CEL were funded by NSF DEB grants 1120688 and 1120652 as well as the Charles A. Dana Foundation. This is Contribution no. 230 to the Bermuda Biodiversity Project ( BBP) of the Bermuda Aquarium , Natural History Museum and Zoo ( BAMZ), Department of Conservation Services .
| M |
Botanische Staatssammlung München |
| T |
Tavera, Department of Geology and Geophysics |
| R |
Departamento de Geologia, Universidad de Chile |
| I |
"Alexandru Ioan Cuza" University |
| MICH |
University of Michigan |
| A |
Harvard University - Arnold Arboretum |
| B |
Botanischer Garten und Botanisches Museum Berlin-Dahlem, Zentraleinrichtung der Freien Universitaet |
| P |
Museum National d' Histoire Naturelle, Paris (MNHN) - Vascular Plants |
| L |
Nationaal Herbarium Nederland, Leiden University branch |
| LSU |
Louisiana State University - Herbarium |
| C |
University of Copenhagen |
| CEL |
University of Illinois |
| BAMZ |
Bermuda Aquarium, Museum and Zoo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.