Drosophila setifemur Malloch, 1924
|
publication ID |
https://doi.org/10.3853/j.0067-1975.61.2009.1517 |
|
persistent identifier |
https://treatment.plazi.org/id/873587D9-0174-B72C-FF47-862CB5B8FEC2 |
|
treatment provided by |
Carolina |
|
scientific name |
Drosophila setifemur Malloch, 1924 |
| status |
|
Drosophila setifemur Malloch, 1924 View in CoL
Drosophila setifemur Malloch, 1924 View in CoL , Proc. Linn. Soc. N.S.W. 49: 351. Holotype ♀ and 1 paratype ♀ in AMS, 2 paratype ♀♀ in USNM; type locality Sydney , New South Wales, Australia.
Not Drosophila setifemur sensu Clark, 1957 View in CoL , Aust. J. Zool. 5: 216–222; Mather, Baimai & Bock, 1969: 72; Wilson et al., 1969: 215–216.
Not Drosophila sulfurigaster (Duda, 1923) View in CoL Spinulophila, Annls hist.-nat. Mus. natn. hung. 20: 48; Wilson et al., 1969: 215–216.
Drosophila (Sophophora) dispar Mather, 1955 View in CoL , Aust. J. Zool. 3: 570 (and as redescribed by Bock, 1976: 19). Holotype 3 in AMS, 24 paratypes, ex type culture, in AMS (including specimens once in SPHTM), ANIC, BMNH, QM, USNM; type locality Samford, near Brisbane, Queensland. New synonym.
Type material examined. Drosophila setifemur female holotype ( AMS K50090 View Materials , registered 20 September 1924) and one female paratype (also registered AMS K50090 View Materials , but given a replacement number AMS K 118452 in 2005) (two female paratypes in USNM [see Malloch, 1924; Lee et al., 1956] not examined). Drosophila dispar , holotype 3, allotype, 433 and 4♀♀ paratypes ( AMS K67819 View Materials – K67824 View Materials , K233649–K233652) ( 16 paratypes in the following museums [233 and 2♀♀ in each according to Mather, 1955: 547]: ANIC, USNM, QM, and BMNH not examined); all ex type culture, founded from one or several females collected at Samford, southern Queensland, 22 June 1953, W.B. Mather. These are holotypes and paratypes (see statement in preamble of Mather, 1955: 547) not syntypes as indicated by Daniels ( Daniels, 1978: 440).
Other material examined. Numerous specimens of Drosophila setifemur (previously det. D. dispar by Mather, Bock, McEvey, Parsons, McAlpine), have been examined in the Australian Museum, the following is a list of 1923–2008 collecting localities arranged from lowest to highest latitude along Australia’s east-coast, collectors include D. McAlpine, P. Parsons, C. Lambkin and S. McEvey: 12, Mt Bellenden Ker, 17.27°S (northern-most record); 2, Lake Eacham, 17.28°S; 3, The Crater NP, 17.42°S; 1, Laceys Creek, 17.85°S; 5, Paluma, 19.01°S; 1, Mt Dalrymple Rd, 21.13°S; 2, Mary Cairncross Park, 26.80°S; 1, Bunya Mountains, 26.85°S; 9, Mt Glorious, 27.33°S; 22, Samford, 27.37°S; 3, Joalah NP, 27.90°S; 1, Tamborine Mountain, 27.92°S; 3, Cunninghams Gap NP, 28.05°S; 120, Lamington NP, 28.14°S; 3, Binna Burra NP, 28.18°S; 1, Bilambil, 28.22°S; 3, Mt Warning NP, 28.40°S; 6, Toonumbar SF, 28.47°S; Tooloom Range, 28.48°S; Dome Mountain, 28.48°S; 1, Huonbrook, 28.53°S; 1, Whian Whian SF, 28.60°S; 8, Terania Creek, 28.67°S; Richmond Range, 28.81°S; Gibraltar Range, 29.47°S; Lowanna, 30.07°S; 1, Moonpar SF, 30.22°S; Bruxner Park, 30.24°S; 3, Dorrigo NP, 30.33°S; 1, Dingo Tops FP, 31.65°S; 1, Upper Allyn River, 32.13°S; Wootton, 32.31°S; 100+, Stroud garden, 32.41°S; 2, Mungo Brush, 32.53°S; 1, Palm Grove, 33.33°S; 4, Mount Wilson, 33.50°S; 1, Kurrajong, 33.55°S; 1, Mt Boyce, 33.62°S; 6, Springwood, 33.70°S; 3, Sydney, 33.88°S; 3, Palm Creek, 34.10°S; 5, Royal NP, 34.10°S; 1, Otford, 34.22°S; 2, Mt Keira, 34.40°S; 6, Mt Saddleback, 34.68°S; 3, Kangaroo Valley, 34.73°S; 1, Monga, 35.58°S; 10, Boyds Creek, 37.43°S; 1, Kinglake, 37.53°S; 1, Naghi SF, 37.55°S; 1, Hurstbridge, 37.63°S; 1, The Narrows, 37.88°S 147.97°E; 1, Ferntree Gully, 37.88°S 145.30°E (southern most Australian record, and western most Victorian record).
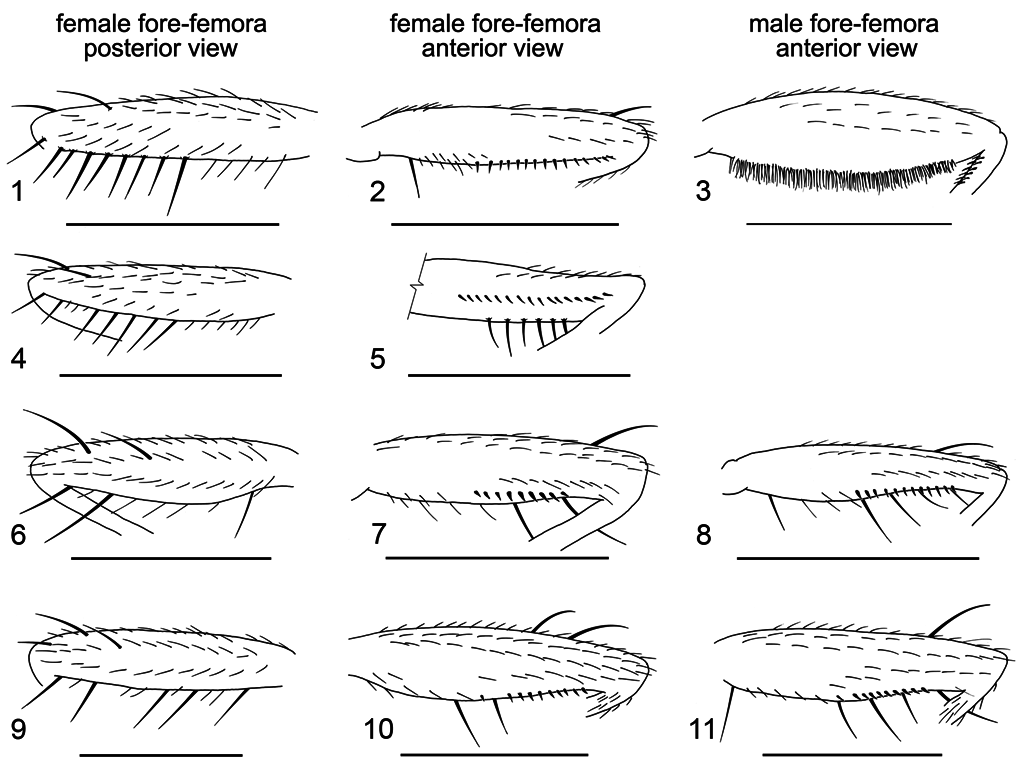
Redescription (based on Drosophila setifemur —McEvey Reg 25302, AMS K259065 male). Carina prominent but relatively narrow, ridged (ridge narrower in males, slightly broader in females). Thorax uniformly mid to dark brown. Male fore-femur plump with dense brush of erect hairs below; fore-metatarsus with a single, short, weak, curved, apical tooth; second tarsal segment with a similar tooth. Female fore-femur lacking dense brush and not unusually swollen; fore-tarsi without apical teeth. Abdomen glossy, blackish-brown, pale basally becoming black apically.
Body length. c. 2.5 mm.
Head. Arista with 4–5 branches above and 3 below plus terminal fork. Front very slightly broader than long, shining yellowish-brown; third antennal segment brownish; periorbital bands enclosing orbital and vertical bristles darker; ocellar triangle darker. Facial carina sharp and high, not broadened nor flattened below, lowest between antennal segments, highest and slightly pointed, in middle of face. Lower part of face distinctly darker than gena. Gena linear, pale; greatest width 0.1 greatest diameter of eye. Vibrissae duplicated, second about 0.9 of first. Eyes with dense fine pile. Orbital bristles in ratio 4: 1: 4; anterior reclinate fine, about equidistant between the proclinate and posterior reclinate, and slightly lateral to proclinate.
Thorax. Uniformly mid to dark brown, not vittate; lower part of postpronotum slightly paler.Acrostichal hairs in 8 irregular rows in front of dorsocentral bristles, 4–6 rows between dorsocentrals. Ratio anterior to posterior dorsocentrals 0.6. Prescutellars absent. Sterno-index 0.5–0.6. Legs pale yellowish-brown. Sex-comb of male consists only of 2 weak and slightly curved teeth, 1 apically on metatarsus, the second apically on 2nd tarsal segment ( Bock, 1976: 19); because these teeth are so weakly developed, Mather (1955) is perhaps justified in describing this condition as “no sex comb”. Both Bock (1976: 19) and Mather (1955: 571) refer to preapical bristles being on all tibiae; and apicals only on the mid-tibia, but I can find no clearly differentiated preapical setae on the fore tibiae in any of the males examined in this study.
Male fore-femur: plump, with numerous, fine, erect setulae brush-like on entire lower surface ( Fig. 3 View Figures 1–11 ); anteroventrally with no seriate spinescent setulae ( cf. sulfurigaster males and females, compare Fig. 3 View Figures 1–11 and Figs. 8, 11 View Figures 1–11 ).
Female fore-femur: posteroventrally with a row of setae in slightly more than apical half ( Figs. 1 and 4 View Figures 1–11 ), all setae subequal in length, evenly spaced and shorter than or equal to the femoral diameter. In describing the holotype female Malloch wrote (1924: 351), and it is here confirmed, that the “fore-femur [is] with short closely placed fine setulae on more than the apical half of posteroventral surface, the longest one, at apex, not longer than the femoral diameter”. This is not a reference to the seriate spinescent setulae on the anteroventral surface. Female fore-femur not swollen, without brush of erect setulae below as in males (compare Fig. 2 and 3 View Figures 1–11 ); anteroventrally with seriate spinescent setulae ( Figs. 2 and 5 View Figures 1–11 ).
Abdomen glossy, blackish-brown, paler basally, becoming black apically. Halteres yellow.
Wings ( D. setifemur holotype AMS K50090 View Materials ). Hyaline. C -index = 2.38; 4v -index = 2.04; 5x -index = 1.76; 4c -index = 1.12; ac -index 2.57; M -index = 0.61; third costal section with fringe of heavy setation on basal 0.67. Length, from humeral crossvein to apex, 2.2 mm (from axis to apex, c. 2.5 mm).
Male terminalia. Cercus very small with very long finger-like process extending from below ( Mather, 1955, fig. 11C; Bock, 1976, fig. 9); the latter is translucent (not heavily sclerotized), strongly curved, tapering to a point, and protrudes well outside the body making it, and the opposing one, clearly visible under low magnification. Under high magnification several sensilla are visible on these processes subapically. Aedeagus with prominent subapical ornamentation.
Distribution. Type locality is Sydney, Australia where the species is taken frequently at fruit baits in urban bushland ( Table 1). This species has been collected at numerous sites in eastern Australia (see above) from northern Queensland at the summit of Mt Bellenden Ker ( 1561 m) to Ferntree Gully, outer eastern suburb of Melbourne, Victoria. It apparently does not overlap with the range of D. prodispar Parsons & Bock, 1982 , in western Victoria.
Notes
Drosophila setifemur closely resembles D. prodispar and is therefore probably closely related to it. The structure of the male and female terminalia, the sexually dimorphic fore-femur and the generally dark coloration of these flies make them quite unlike others in the Drosophila subgenus Sophophora .
A variety of different terms have been applied to the spinescent setulae lying in a series along the lower anterior surface of the fore-femur, and there has been a tendency to emphasize only this aspect of the femoral setation, ignoring the taxonomically useful arrangement of long setae on the posteroventral surface. Reference is made, for example, to “short, stout, spine-like bristles on lower apical part of fore femora” (Patterson & Wheeler, 1942); “comb-like series of stout bristles on femur” ( Mather, 1955); “row of … short, stout, microscopic setae on the apical half of the anteroventral surface of the fore femur” ( Clark, 1957); “fore femur with more or less well developed row of short stout comb-like teeth (femoral comb)” ( Bock, 1976); “the comb-like bristle row on the inner side of the first femur” ( Wilson et al., 1969). It is only Malloch (1924) however, who made reference to the diagnostic utility of the setation on the posterior side of the fore-femur. Indeed it is the relative length and arrangement of long setae arising from the lower fore-femur on its posterior side that offers a more definitive means of separating species of the immigrans and setifemur species groups, at least in females. Males of D. setifemur completely lack serial spinescent setulae and have instead a thick brush of erect hairs ( Fig. 3 View Figures 1–11 ).
When comparing Drosophila prodispar and D. setifemur, Parsons & Bock wrote that both “species show the same dimorphism in carina width and hypertrophy of the forefemur. However, examination of the genitalia under the higher powers of a stereo microscope reveals diagnostic differences in both sexes. In the male, the aedeagus is cylindrical in dispar [= setifemur ] but broadly flattened in prodispar ; a long slender curved finger-like process extending from the genital arch [sic] is visible on each side in dispar [= setifemur ], while the corresponding process in prodispar is shorter, wider and barely curved. In the female, the egg guide in dispar [= setifemur ] possesses a slender apical extension bearing fine teeth; in prodispar the egg guide possesses no apical extension and fine teeth are absent. These differences are evident in pinned specimens and should also be obvious in live flies” (Parsons & Bock in Bock, 1982: 53). As specimens of D. setifemur and D. prodispar examined by me in the AM have a distinctive extension of the cercus, not the genital arch, I believe the above reference to an extension of the “genital arch” (epandrium) is an error. Note also that sexual dimorphism in the width of the carina is rather subtle.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Drosophila setifemur Malloch, 1924
| McEvey, Shane F. 2009 |
Drosophila setifemur sensu Clark, 1957
| Wilson, F & Wheeler, M 1969: 215 |
Drosophila sulfurigaster (Duda, 1923)
| Wilson, F & Wheeler, M 1969: 215 |