Entedon costalis Dalman, 1820
|
publication ID |
https://doi.org/10.11646/zootaxa.1964.1.2 |
|
persistent identifier |
https://treatment.plazi.org/id/88298793-FFA1-FFCE-FF67-FA0BA952F853 |
|
treatment provided by |
Felipe |
|
scientific name |
Entedon costalis Dalman, 1820 |
| status |
|
Entedon costalis Dalman, 1820 View in CoL
Entedon costalis Dalman, 1820: 174 View in CoL .
Tranocera costalis Curtis, 1829: 116 View in CoL (new combination for Entedon costalis View in CoL ).
Eulophus discolor Nees, 1834: 172 View in CoL .
Elachestus costalis (Dalman) View in CoL , Nees, 1834: 143.
Pleuropachus costalis (Dalman) View in CoL , Westwood, 1837: 436 (new combination for Entedon costalis View in CoL ).
Eulophus martialis Förster, 1841: 42 View in CoL .
Eulophus discolor Nees, Dalla Torre, 1898: 29 View in CoL (possible synonym of Pleuropachus costalis View in CoL ).
Eulophus martialis, Dalla Torre, 1898: 29 View in CoL (new synonym of Pleuropachus costalis View in CoL ).
Entedon costalis punctatus Erdös, 1944: 18 View in CoL .
Type material. Lectotype ♂ ( Fig. 1A, B View FIGURE 1 ) and paralectotype ♂ (both on the same pin), Sweden ( Naturhistoriska Riksmuseet , Stockholm, Sweden).
Additional material: 2 ♀, 3 ♂, Kyiv, Park Druzhby Narodiv (plot 4, Table 1), 24.vi.1995, swept from Taraxacum officinalis agg., inflorescence ; 1 ♀, 2 ♂, 06.vi.2006, Kiev, “ Solomyanka ” (plot 5, Table 1), ex earthen cells of G. punctiger ; 7 ♀, 7 ♂, Kiev, Lysa Hora (plot 3, Table 1), ex earthen cells of G. punctiger , 9–14.v.2006 ( Schmalhausen Institute of Zoology of Ukrainian Academy of Sciences, Kiev, Ukraine) ; 1 ♀, 3 ♂, Danemark, 16/5 97 [“ Tekkekjib ” handwritten], coll. Schlick ; 6 ♀, 9 ♂, ibid .; 1 ♂, 18/5 90, ibid .?; 1 ♂, 2 ♀, 12/5 95, ibid .; 1♂, 9/5 86, ibid .?; 2 ♂, 13/5 92, ibid .?; 4 ♀, 22/5 86, ibid .?; 1 ♀, 18/5 90, ibid .?; 4 ♂, 16/5 97, ibid. (collection of the Zoological Museum , University of Copenhagen, Danmark) ; many non-accounted specimens in the collections of Természettudomanyi Museum Allatara ( Budapest, Hungary), and the Natural History Museum ( London, UK) .
Recent literature. Entedon costalis has gained little attention since its description ( Dalman 1820). Apart from some regional records ( Erdös 1944; Graham 1963; 1971), this species was mentioned in comparative diagnosis of E. marginalis , which is similar in structure of fore wing of male ( Askew et al. 2001).
Comparative notes. Within the European fauna, males of E. costalis are easily recognizable in having notably expanded marginal vein ( Fig. 1C, D View FIGURE 1 ). In this respect E. costalis resembles E. marginalis , but the males differ in the structure of antennae ( Askew et al. 2001). Females also possess the expanded marginal vein, which distinguishes them from the females of most other species. Females of E. costalis and E. marginalis differ mostly by size (about 3.5 mm in E. costalis , and about 1.9 mm in E. marginalis ). In all morphological aspects females of E. costalis resemble females of E. pseudonigritarsis Erdös , from which it barely differs, except the marginal vein is broadened throughout in E. costalis and lower margin of clypeus is more notably produced in E. pseudonigritarsis . Males of E. pseudonigritarsis are distinctly different from males of E. costalis in having notably narrower marginal vein (not so distinctly thickened as in E. costalis ). Apart from these morphological differences, E. pseudonigritarsis is a good biological species, having a different host (a weevil Coryssomerus capucinus (Beck 1817) on scentless chamomile Tripleurospermum perforatum, Hinz & Müller-Scharer 2000 ).
Distribution. Austria, Switzerland, former Czechoslovakia, former Yugoslavia ( Bouček & Askew 1968); Croatia, Norway ( Bouček 1977), Czech Republic, Slovakia ( Kalina 1989); Germany ( Förster 1841, Nees 1834, Vidal 2001); Hungary ( Erdös 1944, 1956); Russia ( Yefremova 2002); Sweden ( Dalman 1820, Hansson 1991), Ukraine (new record).
Biology. Egg-larval parasite of dandelion weevil Glocianus punctiger .
Ecology. The host. Glocianus punctiger ( Fig. 6A View FIGURE 6 ) is one of only a few weevils attacking dandelions in Europe (Scherf 1963, Honĕk et al. 2005, Honĕk & Martinková 2005). This weevil was established in biocontrol programs against dandelions in North America ( McAvoy et al. 1983). Likewise many ceutorhynchine weevils, Glocianus punctiger has underground life span; the biology of the weevil is described below in order to facilitate understanding of the parasitoid-host relationships of Entedon costalis .
Females of G. punctiger oviposit in April and May, in synchrony with flowering of dandelions. The female weevil lays its eggs into the dandelion flowerstalk, through a hole cut by the female’s mandibles. Oviposition sites are traced by brown drops of oxidized sap released as a result of damage caused by the female weevil. The eggs are deposited on the internal surface of the flowerstalk ( Fig. 6B View FIGURE 6 ) and are either not attached and simply adhered to the moistured inner flowerstalk surface, or glued there by the hardened plant's sap ( Fig. 6B View FIGURE 6 ). The egg is about 0.8 mm long and 0.5–0.6 mm wide, ovoid ( Fig. 6C View FIGURE 6 ), but occasionally conical ( Fig. 6D View FIGURE 6 ). The embryo develops within about 3–4 days. The newly hatched first instar is pale-yellow, its head capsule is about 0.9–1.0 mm long, pale, but darkens soon. The head capsule of the first instar larva is darker than the head capsules of the older instars. Upon eclosion, the first instar crawls upwards to the receptacle ( Figs 6E, F View FIGURE 6 ). It begins the consuming the receptacle and molts to the second instar. These instars are found in the prereceptacle cavity, within the receptacle plate, or among the young seeds. The larva consumes young seeds, and moults to the third (final) instar shortly before maturation of the seeds. As soon as the mature seeds disperse the mature larvae are revealed, fall to the ground and quickly bury into the soil. There they prepare an ovoid earthen cell, about 3.0 mm long, pupating therein about one week later ( Fig. 14C, D View FIGURE 14 ). In Kiev, the mature larvae of G. punctiger drop out from the seed heads of dandelion from early May through early June, leaving darkened orifices indicating their injuries to the host plant. Pupae can be found in earthen cells about 5–8 days after larvae fall to the ground, and adult beetles emerge about two weeks later (early to late June). Emerged beetles feed on dandelion rosettes during rest of summer and overwinter in the soil. Honek et al. (2005) reported that larvae of this weevil were found in Czech Republic until September, associated with continual flowering of dandelions in some patches.
Ovipositing behaviour of E. costalis . The females of E. costalis can be found in the field in the north of Ukraine ( Kiev) for a very short time, from late April until mid May. The parasitoid female searches along the dandelion flowerstalk by drumming its surface with her antennae. It is very likely that she locates the host by the hardened host-plant sap ( Figs 4A, B View FIGURE 4 , arrowed), in places of penetration of the weevil’s rostrum. Once the female has located a hardened sap, she starts more frequent drumming and soon afterwards starts probing the flowerstalk by her ovipositor ( Fig. 4A, C View FIGURE 4 ). She bends her gaster downwards, briefly hooks the ovipositor saw into the plant tissues and then releases the gaster so that it strengthens in a position perpendicular to the ovipositor. Thereafter she penetrates the flowerstalk wall and presses the gaster downwards up to the plant surface ( Fig. 4B, D View FIGURE 4 ). During this time she moves the ovipositor inside the flowerstalk and probably searches for the host egg inside. If the egg is not found, or occasionally by some other reason, she withdraws the ovipositor from the host egg and continues searching. The probing normally lasts 8–15 seconds. If the egg is found, the ovipositing starts. The female moves like in process of probing, but keeps the ovipositor more deeply inside the flowerstalk, in average about 45–50 seconds. During all this time the female carries out rhythmic, twisting movements of her gaster ( Fig. 4D View FIGURE 4 ).
Parasitoid-host relations. Entedon costalis is an egg-larval parasitoid of G. punctiger . The parasitoid eggs are free floating within the egg yolk and are occasionally discernible there ( Fig. 7A View FIGURE 7 ). Generally E. costalis lays eggs singly, but deposition of more than one parasitoid egg per one host egg (superparasitism) was encountered in several cases (e.g. Table 2, Fig. 9 View FIGURE 9 ). Both unparasiztized and parasitized eggs of G. punctiger are yellowish, but the parasitized eggs bear dark round markings which are in all likelihood the melanized points of the parasitoid ovipositor's penetrations ( Fig. 7B View FIGURE 7 ). Occasionally brownish eggs with larger melanized markings occur. These eggs become soon dark brown, contain undifferentiated mass instead of embryo and decay. The dissections of the eggs with large melanized markings revealed parasitoid eggs surrounded by aggregations of cells (putatively haemocytes, Figs 7C, D View FIGURE 7 , hm).
The parasitoid egg is passively encompassed into weevil's embryo and the parasitoid first instar hatches within the body of the actively feeding first instar of the weevil, generally when the latter approaches the food plant receptacle ( Figs 6E, F View FIGURE 6 ). Parasitoid eggs inside developing embryos were found free-floating in the body cavities of the host first instar. The eggs and newly hatched larvae, where detectable, were localized near the larva's mid gut ( Fig. 9 View FIGURE 9 ). Parasitoid larvae hatch after the host’s hatch, about 5–7 days after oviposition. About one day later the first instar parasitoid starts eating. Simultaneously the rival larvae are killed in the case of superparasitism ( Figs 10A, B View FIGURE 10 ). The parasitoid first instar goes through the host’s first moult and moults to the second instar ( Figs 12A, B View FIGURE 12 ) already inside the body of the second instar weevil larva. This larva ( Figs 12B, C View FIGURE 12 , 13 View FIGURE 13 ) goes through the host's second moult and moults to the final instar ( Figs 14A, B, E View FIGURE 14 , 15 View FIGURE 15 ) already inside the body of the mature (third instar) weevil larva. Both parasitized and unparasitized weevil larvae actively feed on young dandelion seeds, fall to the ground after feeding, bury themselves and successfully construct earthen cells underground ( Figs 14C, D View FIGURE 14 ).
Subsequently, parasitized larvae do not pupate as do unparasitized larvae. Rather, they remain immobile for 13–16 days, during which the host is consumed by the final parasitoid instar. The fully fed parasitoid larva leaves the host’s remnants ( Fig. 14E View FIGURE 14 ) and pupates in 2–3 days. The parasitoid pupae ( Fig. 14F View FIGURE 14 ) may be found inside the host’s earthen cells or directly in soil (if the host failed to construct the cell). Adult parasitoids emerge the following spring.
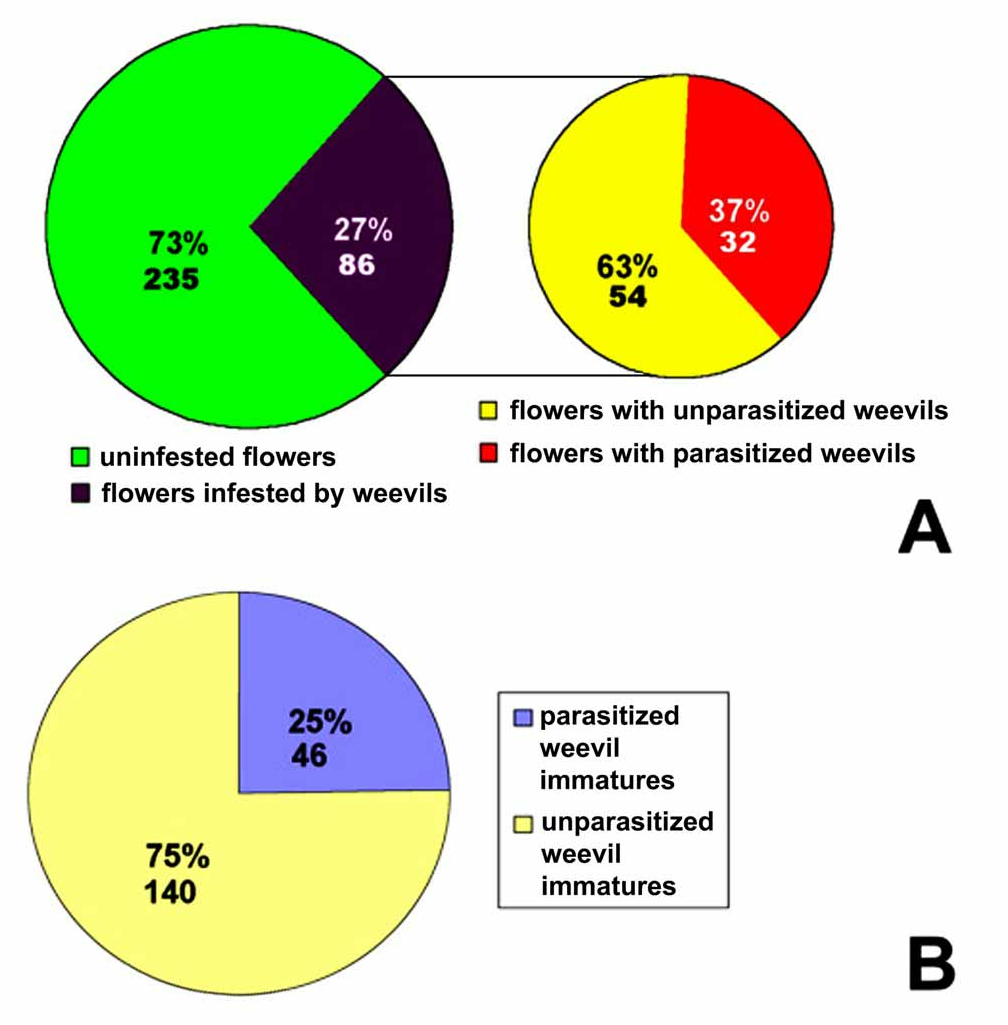
Tri-trophic relationships. Table 2 provides comparative data on ratio of parasitized and unparasitized weevil instars in each given flower infested by weevils. Table 2 is based upon analysis of data of the chosen model patch ( Table 1, plot 6, sampled in May 8 2006) where dandelion flowers were relatively evenly distributed, and relatively high infestation of dandelion inflorescence by weevils and of weevil immature stages by E. costalis was found ( Table 2). Of 321 dandelion flowers collected in this patch, 86 flowers (27 %) contained either eggs or larvae of G. punctiger . Of these, 32 flowers (10% of the total flowers collected and 37 % of infested flowers, respectively) contained the parasitized weevils at various stages of development. Total degree of parasitism of G. punctiger by E. costalis was 25 % in the model patch ( Table 2, Fig. 5 View FIGURE 5 ). The host weevil was simultaneously represented by the eggs, first and second larvae, and the parasitoid was represented by the eggs and first instars.
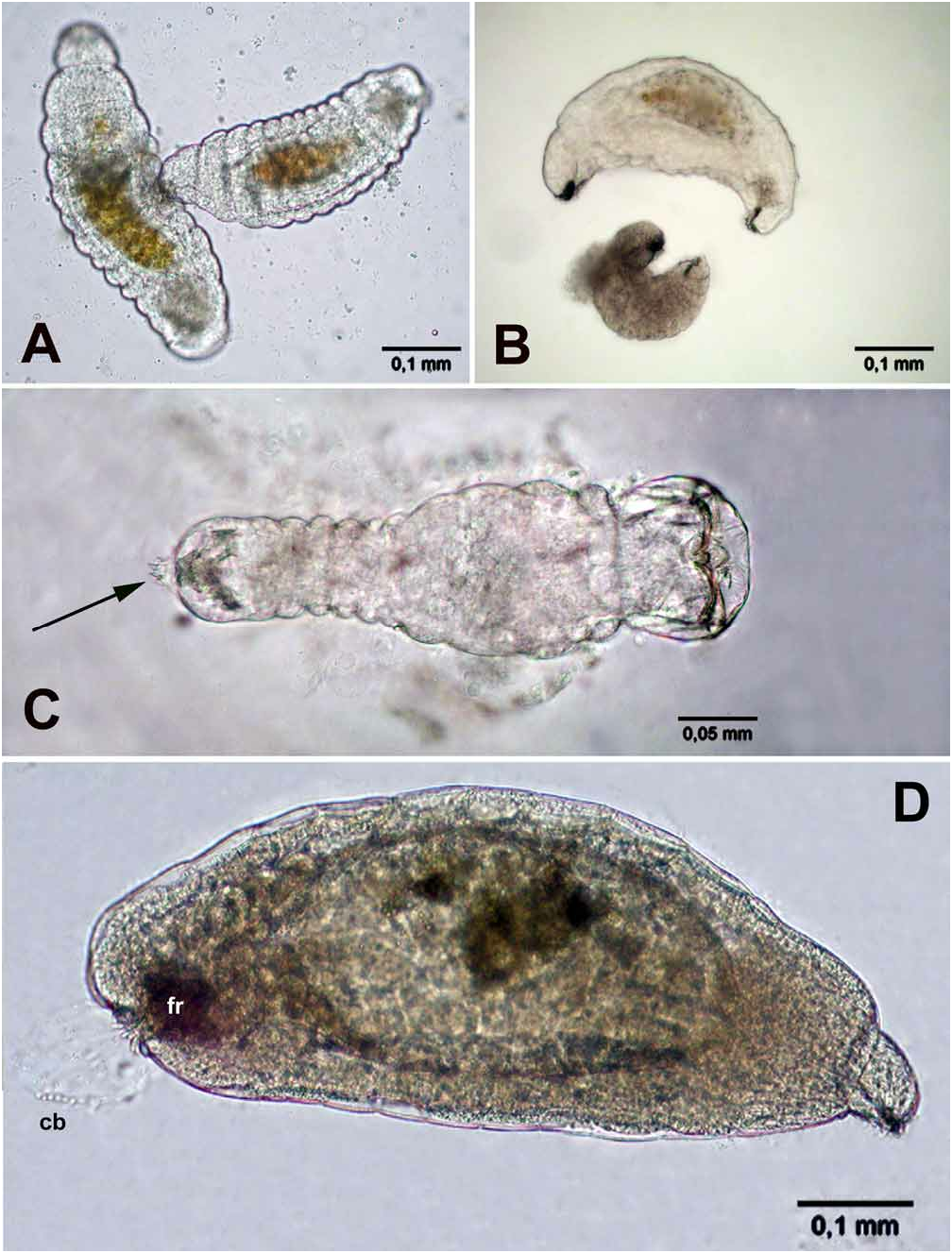
Superparasitism. Superparasitism was observed in 6 of more than 1000 total dissections of weevil larvae. Mechanical siblicide was observed in one case ( Figs 10A View FIGURE 10 ). The wounded siblings were occasionally found ( Fig. 10B View FIGURE 10 ) and only one second instar parasitoid was ever found per second instar host. These observations suggest frequent siblicide in E. costalis . In one case, five parasitoid specimens were found in the same host first instar ( three eggs and two larvae, Fig. 9 View FIGURE 9 ), but no physical damage of the rival larva and eggs was observed. However, the only one parasitoid larva was actively moving, whereas the other larva was immobile. This may suggest that siblicide occurs not only by physical, but also by physiological suppression ( Vinson & Hegazi 1998, Uka et al. 2006).
Multiparasitism. Another parasitoid was reared from the pupa of G. punctiger , Tersilochus sp. (Ichneumonidae) , one female emerged from an earthen cell. The adults of Tersilochus spp. and Schizoprymnus spp. (Braconidae) were recovered in field while sweeping on dandelions. Some peculiar first instar parasitoids ( Figs 10C View FIGURE 10 ) were found in the first instar larvae of G. punctiger . These larvae were surrounded by serosal membrane, and resembled larvae isolated by the author from Mordellistena falsoparvula ( Coleoptera : Mordellidae ) parasitized by Schizoprymnus sp. (Gumovsky, unpubl.). Honek et al. (2005) reported Schizoprymnus obscurus (Nees) from G. punctiger . This record and possession of the serosal membrane (inherent to many braconid wasps) may suggest that these enigmatic endoparasitoid larvae also belong to either Schizoprymnus , or related braconid. These larvae also have the large sickle-shaped mandibles and serrated caudal appendages ( Figs 10C View FIGURE 10 , arrowed), similar to larvae of Entedon . However these larvae are different in having notably larger and wider head (narrower and “beak”-shaped in E. costalis ), distinct separation to wider thoracic and narrower abdominal segments (not evident in E. costalis ), and narrow caudal appendage. Many solitary parasitoids have mandibulate first instars, but these larvae are generally not covered by a serose membrane. In some parasitoids rivals are eliminated by “soldier” precocious larvae, which physically attack the competitors, but then die without pupation ( Cruz 1981, Grbic et al. 1992). However, no extra eggs or parasitoid embryos were observed in the eggs infested by these enigmatic larvae, what suggests that these larvae are rather solitary reproductive larvae, than precocious soldiers.
Emergence behaviour of parasitoids. Two males and two females, which emerged recently from the host's earthen cells, were used in laboratory experiments on the emergence from soil of adult parasitoids. Newly emerged wasps dig through a soil layer averaging 60 mm in about 60 seconds ( Table 4). The insects push off the surrounding soil particles using their legs, and then move the soil particles aside using both the produced lower margin of the clypeus and mandibles ( Figs 2A, B View FIGURE 2 ). Occasionally, the mandibles were used to pulverize large soil aggregations. Occasionally, the excavated tunnel of the insects was "licked" with the labiomaxillary complex ( Fig. 2C View FIGURE 2 ).
Immature stages of E. costalis
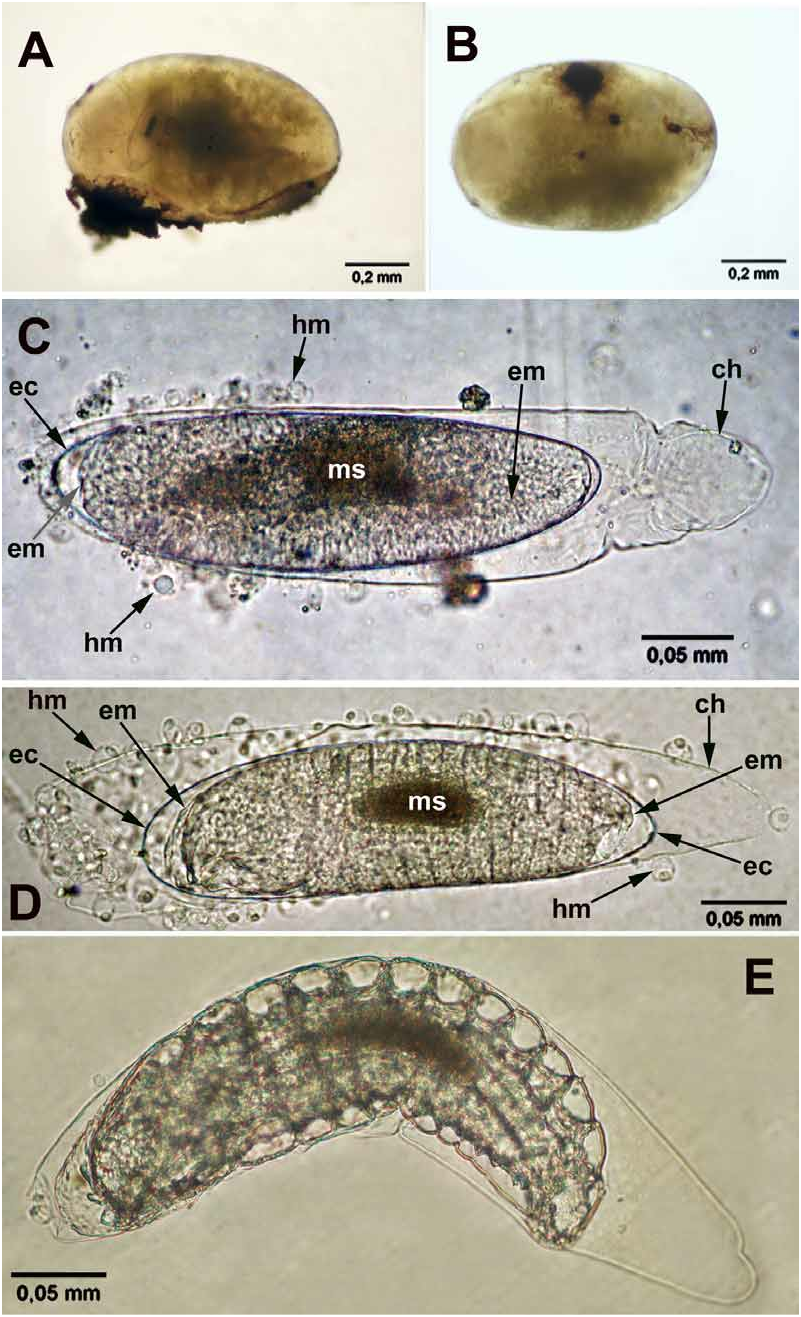
Egg. The eggs isolated from ovaries and non-developing eggs isolated from the hosts are filled with yolk, appear milky and lack discernible sculpture in reflected light, about 0.3 mm long and about 0.05 mm wide. The developing eggs isolated from the host eggs and young larvae are about 0.4 mm long and about 0.1 mm wide. The developed eggs differ from ones isolated from ovaries in having: (1) a darker longitudinal middle stripe (whitish in reflected light, darker in transmitted light microscopy, Fig. 7C, D View FIGURE 7 , ms), which occasionally allows discernment of the egg inside the host ( Fig. 7A View FIGURE 7 ); (2) a compressed chorion posteriorly, which is separated from the embryo ( Fig. 7C View FIGURE 7 , ch). Also, there is an embryonic cuticle ( Fig. 7C, D View FIGURE 7 , ec) between the developing embryo (em) and egg chorion (ch), what is similar to that described by Jackson (1964) for Mestocharis bimacularis (Dalman) (Eulophidae) .
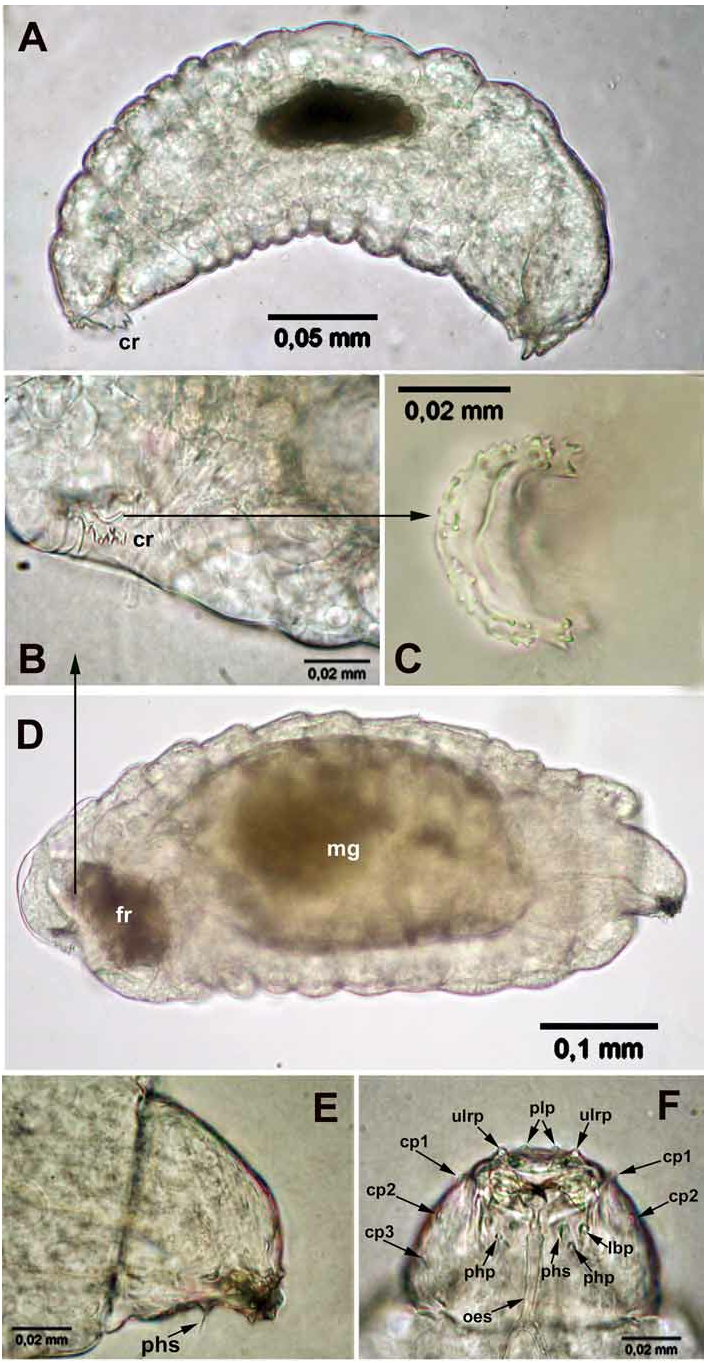
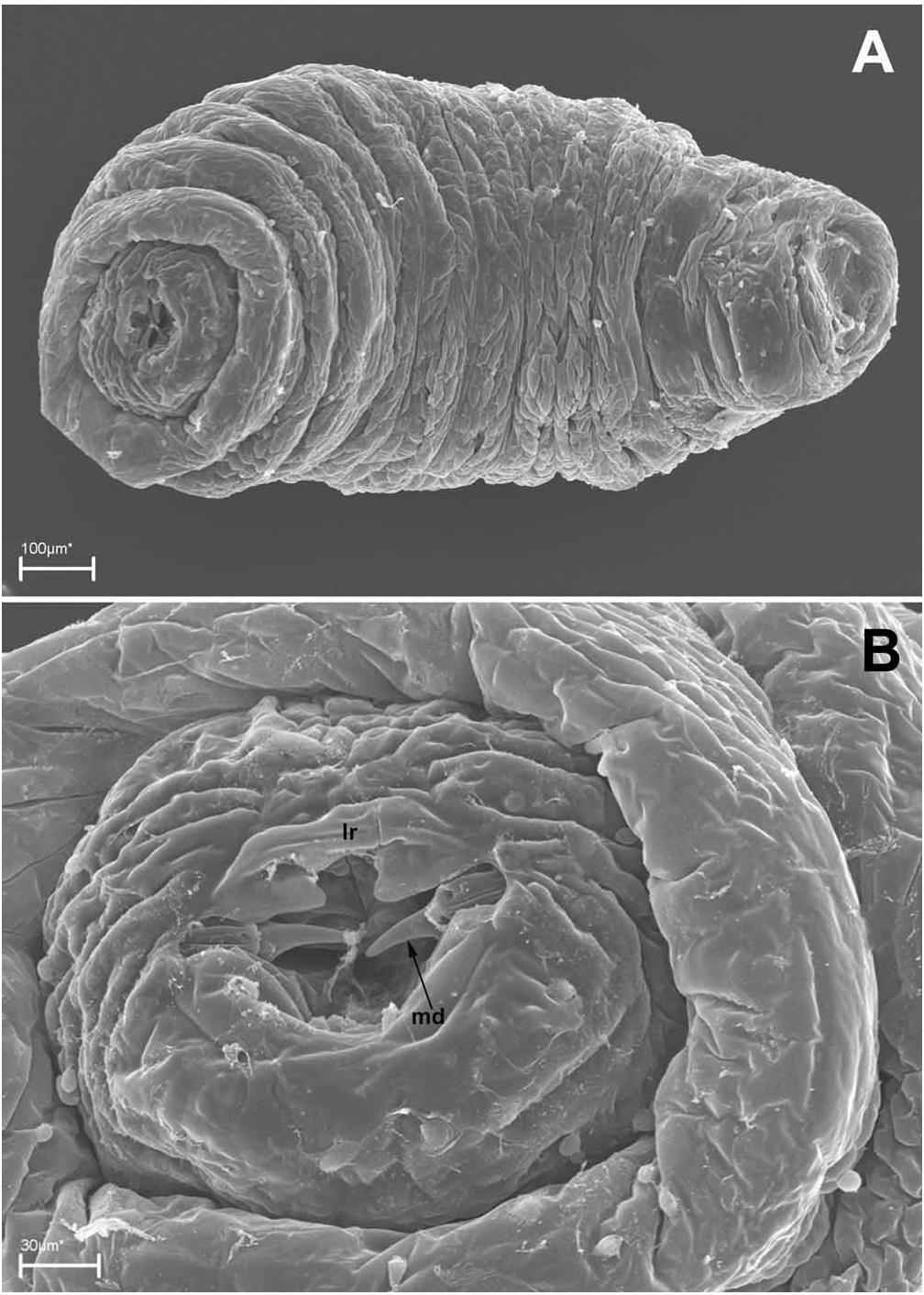
First instar. The first instar is hymenopteriform, pale (occasionally transparent, or with a whitish contents of the mid gut), has 13 body segments and a cranium ( Figs 8A, D View FIGURE 8 , 11A, B View FIGURE 11 ). The young larva starts moving yet inside the egg ( Fig. 7E View FIGURE 7 ). The newly hatched larvae ( Fig. 8A View FIGURE 8 ) are slender, about 0.3 mm long and about 0.1 mm wide. The terminal segment bears sharp triangular tubercles along its dorsal margin, arranged in groups of two or three, or in tufts, and in the general shape of a half-circular crown ( Figs 8A–D View FIGURE 8 ). Segments I–III (thoracic) are bare, whereas segments IV–XII (abdominal) bear distinct dorsal semicircular serrations along their anterior margins, which consist of small teeth ( Fig. 11A View FIGURE 11 , inset). No spiracles were found.
The actively feeding larvae isolated from first- and second instar hosts are larger, about 0.6 mm long and 0.25 mm wide ( Figs 9E View FIGURE 9 , 13A, B View FIGURE 13 ), but still recognizable as representatives of the first instar through the possession of the pecuiar cranium ( Fig. 8D View FIGURE 8 ) and the "caudal crown", but the latter is contracted into the body ( Fig. 8D View FIGURE 8 ). These larvae possess non-sclerotized caudal bladder representing probably an everted hind gut, likewise in other Entedon species ( Gumovsky, 2007) . The bladder is transparent and is hardly visible in living specimens, but appears as a short knob after adding ethanol ( Fig. 10D View FIGURE 10 , cb). The tiny filaments, which are discernible within the bladder emanate from a dark “caudal formation” ( Figs 8D View FIGURE 8 , 10D View FIGURE 10 , fr).
Head capsule ( cranium), Figs 8E, F View FIGURE 8 , 11C, D View FIGURE 11 . The head capsule is weakly sclerotized, narrowing ventrally, with a characteristic “beak”-shaped end, which is formed by the protruding palpi of the labrum, which covers thin mandibles. Antennae (ant) are indicated as small swelling on the upper part of the head capsule ( Figs 11C, D View FIGURE 11 ). The mandibles of this instar are sickle-like, about 0.02 mm long, dark brown and sclerotized ( Fig. 8F View FIGURE 8 ). The sensorial structures of the head are well-developed and arranged in a relatively fixed position (very similar to that described by Gumovsky, 2006, 2007). However, the head sensoria are occasionally obscure and best traced by comparison of images obtained by light and scan electronic microscopy. The lateral area of the cranium bears three pairs of cranial palpi: the upper (cp1), the lower (cp2) and the posterior (cp3) ones. There is a pair of enlarged pleurostomal palpi (plp) just above the labrum. The labrum (lr) bears two pairs of labral palpi (grouped as one from each side): the upper lateral labral palpi (ulrp) and the lower lateral labral palpi (llrp). A large palpus is located near each axilla (mxp) and behind them there is a pair of smaller labial palpi (lbp). A pair of comparatively long pharyngeal setae (phs) is situated on their palpi behind the labial palpi. There is a structure which has not been detected by SEM, but distinct on light-microscopy images ( Fig. 8F View FIGURE 8 ) and reported here as the pharyngeal palpi (php).
Second instar. The identity of this instar was revealed by the observed molt of the first instar ( Figs 12A–B View FIGURE 12 ). The second instar ( Figs 12C, D View FIGURE 12 , 13A View FIGURE 13 ) is also hymenopteriform and pale. The newly molted larva is about 0.7 mm long and about 0.1 mm wide ( Fig. 12C View FIGURE 12 ). In comparison with the first instar the second instar larva is more robust ( Figs 12D View FIGURE 12 , 13A View FIGURE 13 ), none of the body segments has serrations, and the last segment bears no visible “caudal crown”. The head is more spherical (without a protruding, “beak”-shaped labrum) and the sensorial structures hardly detectable. Mandibles of this larva are nearly triangular in shape and lightly sclerotized, about 0.06 mm in length ( Fig. 13C View FIGURE 13 ). The caudal bladder is also distinct, emanating from a caudal formation ( Figs 13 View FIGURE 13 , fr), in a shape of cone. This bladder is full of small, actively moving symbionts ( Fig. 13B View FIGURE 13 , mcr), which are believed to be bacteria judging from their size and pecular motility. No spiracles were found, but the gas-containing tracheae are visible.
Final instar ( Figs 14 View FIGURE 14 , 15 View FIGURE 15 ). The final instar is easily distinguishable from the two previous larvae in having developed tracheal system with one pair of spiracles on the second (thoracic) segment and on sixth–eleventh (abdominal) segments ( Fig. 14A View FIGURE 14 ). The spiracles are closed until the larva is mature. The newly molted final instar measures 0.9–1.3 mm long, 0.6 mm wide ( Figs 14A View FIGURE 14 , 15A View FIGURE 15 ). It has lightly sclerotized mandibles and relatively large spherical caudal bladder with traceable filaments inside its wall. In the larger larvae the mandibles are heavily sclerotized and the caudal bladder is flattened or broken. Mature larvae lose the bladder ( Fig. 14E View FIGURE 14 ).
The mature larva has distinct labrum, labium and large and heavily sclerotized mandibles (nearly 0.08 mm long) with robust bases and curved blades ( Figs 14B View FIGURE 14 , 15B View FIGURE 15 ). The sensilla of the head are less distinct than in the first instar larva.
Pupa ( Fig. 14F View FIGURE 14 ) black, with metallic green, blue or iridescent tint, obtect in shape, with distinct outlines of head, mesosoma, metasoma, wings, legs and antennae. The average length is 2.5 mm, the width of the head is about 0.9 mm, of mesosoma 1.2 mm, of metasoma 1.4 mm.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Entedon costalis Dalman, 1820
| Gumovsky, Alex V. 2008 |
Entedon costalis punctatus Erdös, 1944: 18
| Erdos, J. 1944: 18 |
Eulophus discolor Nees, Dalla Torre, 1898: 29
| Dalla Torre, K. W. von 1898: 29 |
Eulophus martialis , Dalla Torre, 1898: 29
| Dalla Torre, K. W. von 1898: 29 |
Eulophus martialis Förster, 1841: 42
| Forster, A. 1841: 42 |
Pleuropachus costalis
| Westwood, J. O. 1837: 436 |
Tranocera costalis
| Curtis, J. 1829: 116 |
Entedon costalis
| Dalman, J. W. 1820: 174 |