Tetragonopterus anostomus, Silva, Gabriel S. C. & Benine, Ricardo C., 2011
|
publication ID |
https://doi.org/10.5281/zenodo.202383 |
|
DOI |
https://doi.org/10.5281/zenodo.5667128 |
|
persistent identifier |
https://treatment.plazi.org/id/8933879A-FFBD-F127-FF10-DF6DB078B3EA |
|
treatment provided by |
Plazi |
|
scientific name |
Tetragonopterus anostomus |
| status |
sp. nov. |
Tetragonopterus anostomus , new species
Table 1 View TABLE 1 , Figs. 1–2 View FIGURE 1 View FIGURE 2
Holotype. MZUSP 108957, 45.4 mm SL, Brazil: Goiás state, Nova Crixás, rio Preto, rio Araguaia basin, S 14°22’18” W 050°39’13”, 26 jul. 2005, L. S. Souza, M. R. S. Melo, C. C. Chamon, and L. M. Sousa.
Paratypes. MZUSP 89295, 5, 34.8–41.0, mm SL. Collected with holotype. LBP 7687, 17, 34.6–38.9, 2 c&s, 32.5–34.0 mm SL, Brazil: Goiás, Cocalinho, Lagoa marginal of rio Araguaia, rio Araguaia basin, S 14°22’36” W 50°40’08.4”, sep. 2009, J. Senhorini.
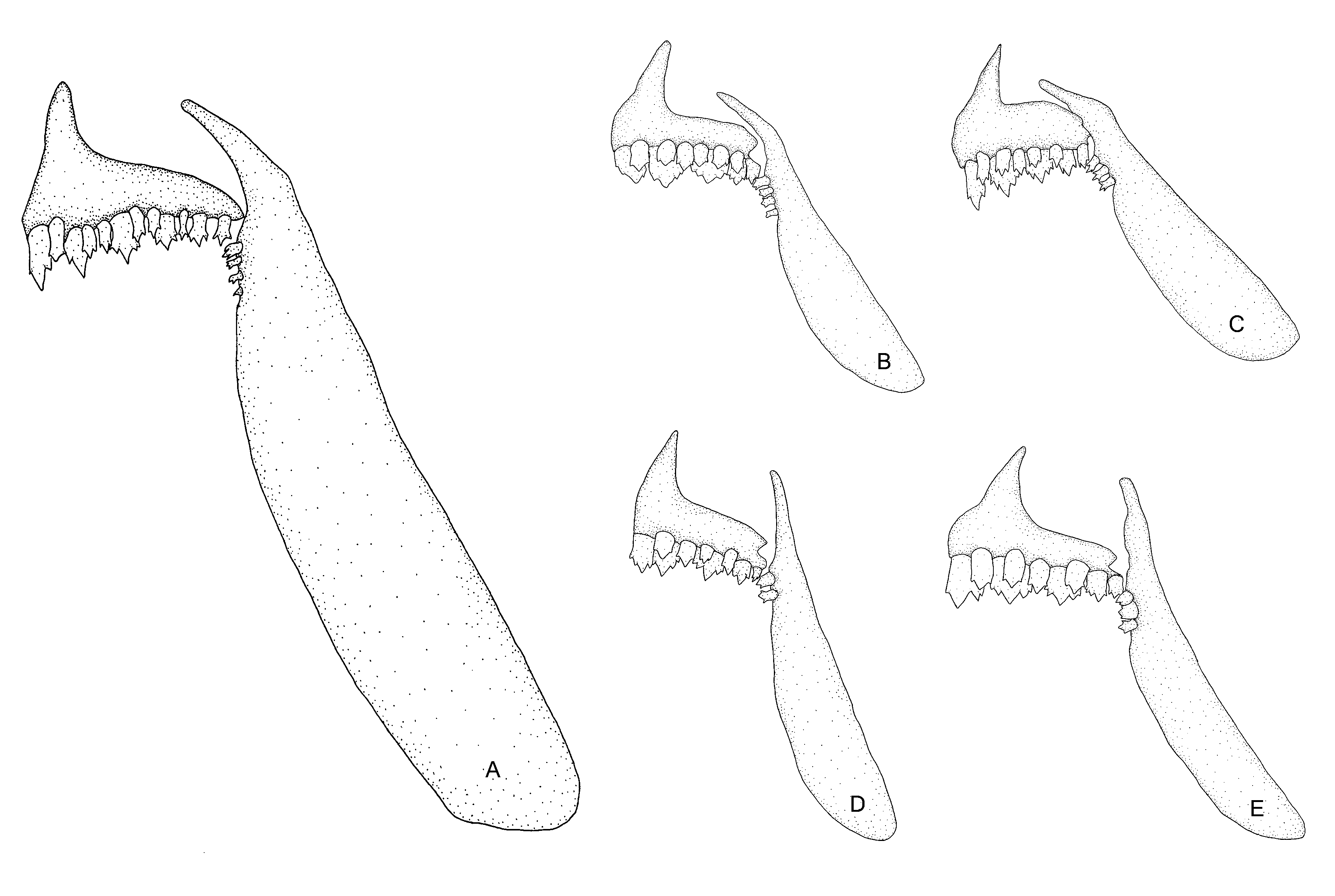
Diagnosis. Tetragonopterus anostomus is distinguished from congeners by having a superior mouth (vs. terminal mouth) with relatively smaller and numerous teeth in the inner row of premaxilla (5–7 vs. always 5, respectively) and dentary (5 vs. 4, respectively), and by the number of gill rakers on the lower limb (18–19 vs. 12–14 respectively) of the first branchial arch. Additionally, T. anostomus is distinguished from congeners by having a comparatively enlarged maxilla (see Fig. 2 View FIGURE 2 for complete comparison). Tetragonopterus anostomus is also distinguished from T. argenteus by the number of predorsal scales (8 vs. 12–17, respectively); from T. rarus by the absence of dark stripes on the lateral surface of the body (vs. presence), and from T. carvalhoi by having a rounded spot vs. a lozenge-shaped spot on caudal peduncle.
Description. Morphometric data of holotype and paratypes are summarized in Table 1 View TABLE 1 . Body compressed, moderately elongated in lateral view. Greatest depth on dorsal-fin origin. Preventral and predorsal area keeled. Dorsal profile slightly convex between tip of snout and vertical through midhalf of orbit; slightly concave from this point to end of occipital process; convex from this point to base of dorsal-fin origin; slightly convex at limits of dorsal and adipose fins. Ventral profile: smoothly convex from snout to slightly behind pectoral-fin origin; convex from that point to anal-fin origin; straight until base of anal fin. Caudal peduncle with dorsal and ventral profiles nearly concave. Snout shorter than orbital diameter. Superior mouth. Upper and lower jaw with similar size. Maxilla straight, aligned at angle of approximately 90 degrees to longitudinal body axis. Premaxilla with two-tooth rows. Both inner and outer rows with relatively small teeth, Outer row with 4* (3), 5 (11), 6 (10) and 7 (1), tricuspid teeth with central cusp slightly longer. Inner row with 5 (4), 6 (19) and 7* (2) tricuspid and pentacuspid teeth with central cusp twice as long and broader than other cusps. Maxilla with 2 (8), 3* (8), 4 (9) conic to tricuspid teeth. Dentary with 5* (13) or 6 (12) tricuspid and pentacuspid anteriormost teeth, followed by 10–11 (2 c&s) smaller conical teeth with similar size, all backward inclined. Dorsal-fin rays ii,9* (25). First unbranched ray smaller than second one. Dorsal-fin origin anterior to middle body; distal border elongated. Dorsal-fin pterygiophores 10 (2 c&s). Anal-fin rays v,26 (1) v,27* (4), v,28 (3), v,29 (8) and v,30 (9); anterior rays usually slightly longer than posterior ones. Anal-fin origin at vertical through base of nine or ten branched dorsal-fin rays. Anal-fin pterygiophores 28–29 (2 c&s). Pelvic-fin rays i,7* (25). Pelvic-fin origin anterior to vertical line through dorsal-fin origin; distal border elongated; tip reaching anal-fin origin. Pectoral-fin i,11 (1) i,12* (16), i,13 (7) and i,14 (1). Caudal fin forked, with i,17,1 rays (2 c&s). Dorsal procurrent rays 13–14 (2 c&s) and ventral procurrent rays 9 (2 c&s). Cycloid scales large. Lateral line complete and distinctly ventrally curved anteriorly. Scales in longitudinal series 29 (4), 30 (3), 31 (12) and 32* (6). Scale rows between dorsal-fin origin and lateral line 6 (13) and 7* (12); scale rows between lateral line and pelvic-fin origin 3* (15) and 4 (10). Scale rows around caudal peduncle 12 (7), 13* (14) and 14 (4). Anal fin base with one scale row, caudal-fin with scales covering part of the lobes. Precaudal vertebrae 8 (2 c&s), intermediate vertebrae 2 (2 c&s) and caudal vertebrae 16 (2 c&s). Supraneural 3 (2 c&s). Lower gill-rakers on first arch 17* (8), 18 (10) and 19 (04); upper gill-rakers on first arch 11* (22).
deviation.
Sexual dimorphism. Bony hooks on anal and pelvic fins, a sexually dimorphic feature widespread among Characiformes (Malabarba & Weitzman, 2003) and known for T. chalceus ( Ricardo et al., 1998) , were not observed in T. anostomus .
Color in alcohol. General body color yellowish. Dorsal portion of head and body dark pigmented. Dorsolateral portion of body with few points of chromatophores on distal edges of scales, scales of ventrolateral portion without pigmentation. Two conspicuous vertical dark marks on humeral region. Anterior humeral mark more evident than posterior humeral mark. Anterior humeral mark, located over four horizontal scales row above lateral line, posterior humeral mark very poor, with cromatophores very scattered. Caudal peduncle with a rounded dark spot. Silver stripe in the medial region of the body, going from the top edge of the opercule to the caudal peduncle. Anal, pelvic, and dorsal fin hyaline, with chromathophores concentrated at the ends of the rays. Adipose fin hyaline. Opercular and infra-orbitals bones silver.
Distribution. Known from the type locality, rio Preto, State of Goiás, Nova Crixás, and lagoa marginal at the municipality of Cocalinho, Mato Grosso, rio Araguaia basin, central Brazil ( Fig. 3 View FIGURE 3 ).
Etymology. The specific epithet is a compound name derived from Greek ano-, meaning upward, above; and stoma, meaning mouth, in reference to the position of the mouth.
Discussion. Tetragonopterus anostomus is easily distinguishable from known congeners as described in the Diagnosis. This new species can be included in the T. chalceus group, which includes species with seven to nine predorsal scales.
Among the 15 nominal species of Tetragonopterus listed as species inquirendae in Characidae by Lima et al. (2003), T. sawa Castelnau was originally described from rio Crixás, rio Araguaia basin, near the type locality of T.
anostomus . Eigenmann (1917) recognized T. sawa as a junior synonym of T. argenteus , a species with 12 to 16 predorsal scales, although neither types nor topotypes were listed by that author as examined material. Eigenmann (1917) redescribed T. argenteus as bearing 12 to 16 predorsal scales, and highlighted that this species “can always be readily distinguished” from T. chalceus , T. gibbosus , and T. huberi “by its great depth and two vertical bars” with no mention of the predorsal scales as a diagnostic character. We were able to examine a photograph of the holotype of T. sawa (MNHN A. 9819) and, even though it was not possible to confirm the number of predorsal scales, it does have a deep body and a terminal mouth ( Fig. 2 View FIGURE 2 ), which promptly distinguishes Castelnau’s species from T. anostomus . These characters also distinguish T. anostomus from Tetragonopterus huberi Steindachner , T. rufipes Valenciennes , and T. schomburgkii Valenciennes , nominal species actually related to the genus.
Even though both T. argenteus and T. chalceus were identified as also occurring in the upper rio Araguaia, only T. chalceus was sampled with T. anostomus from its known area of occurrence (see comparative material). A lot (MZUSP 89295) with both species mixed may be indicative that these species probably school together. Tetragonopterus anostomus and T. chalceus present a very similar, if not identical, overall body color pattern, which would result in a quite homogeneous and mixed school of fishes. If one considers that these fishes school together, among other hypotheses, for reducing the risk of predation ( Chivers et al., 1995; Magurran, 1990), such an interaction between closely related and similar species would possibly be more efficient since it produces larger and more cohesive schools.
Considering such an interaction, T. anostomus has marked modifications in the jaw, teeth ( Fig. 2 View FIGURE 2 ), and gillraker morphology in comparison not only with the sympatric T. chalceus , but with all known congeners. These modifications are intimately associated to feeding habits ( Clabaut et al., 2007), and may represent ecomorphological adaptations for food acquisition within a distinct and less competitive niche. Body size is also related to food acquisition in fishes ( Clabaut et al., 2007) and, as a consequence, differences in this trait may be indicative of exploitation of different niches. In fact, T. anostomus seems to be smaller than T. chalceus , considering that the largest known specimen of T. anostomus does not exceed 45.3 mm SL. Although absolute body size values are not informative, as it may represent straightforward observations of different developmental stages, gonadal analysis indicated that a specimen of T. anostomus (MZUSP 89295) with 38.0 mm SL is a mature female. According to Ricardo et al. (1998), males and females of T. chalceus have their first maturation at no less than 48.0 to 63.0 mm SL. More comprehensive studies on interspecific interactions may shed light on our understanding of the evolution of the group ( e.g. Alexandrou et al., 2011).
Comparative material. Tetragonopterus argenteus : Venezuela: Bolivar: Caicara del Orinoco, rio Orinoco: LBP 3058, 2,61.0–81.2; LBP 3059, 1, 57.1. Brazil: Maranhão: LBP 5535, 1, 74.4, Balsas, rio Balsas. Acre: LBP 185, 2, 71.2–72.5, Rio Branco, rio Acre; MZUSP 50301, 3, 81.8–88.5, Colocação Volta Grande, rio Grande. Mato Grosso; Aripuanã: NUP 3381, 5, 62.9–82.0; NUP 7189, 2 55.6–57.6; Nova Brasilândia: NUP 10768, 1, 101.0, rio Manso, rio Paraguai; Rosário Oeste: NUP 10758, 17, 24.2–44.5, rio Cuiabá, rio Paraguai; Chapada dos Guimarães: NUP 4092, 3, 65.1–87.6, rio Cuiabá, rio Paraguai; LBP 3967, 1, 59.5, Cuiabá, rio Mutuca; LBP 4633, Paconé, rio Paraguai. Goiás; Aragarças; LBP 1832, 1, 68.9, rio Araguaia. Mato Grosso do Sul; Coxim: LBP 1476, 7, 27.4– 42.5, rio Paraguai; LBP 1776, 4, 81.3–97.8, rio Paraguai; Aquidauna: LBP 3758, 20, 50.4–66.7, rio Paraguai; Cáceres: LBP 8442, 3, 51.8–63.9, rio Paraguai; LBP 5108, 1, 67.6, rio Paraguai. Paraná: NUP 7341, 1, 110.9, Foz do Iguaçu, rio Paraná; Peru: MZUSP 26393, 10, 40.8–67.5, Pucallpa, rio Ucayali. Tetragonopterus chalceus : Brazil. Amapá: MZUSP 35008, 6, 59.3–95.5, rio Cupuxi. Pará: MZUSP 92777, 5, 70.4–100.0, Santarém, lago do Maiacas, rio Amazonas; MZUSP 35006, 8, 48.5–54.7; Itabuna, rio Tapajós; MZUSP 35017, 5, 47.9–82.4, São Luís, rio Tapajós. Tocantins: Ananás, rio Tocantins: NUP 8202, 8, 34.43–53.31; NUP 9034, 1, 54.6. Roraima: MZUSP 35004, 36, 43.2–85.7, Rio Branco, rio Branco. Mato Grosso; Cocalinho: LBP 8773, 1, 54.2, MZUSP 89182, 5, 37.6–47.7. Goiás: Nova Crixás, rio Preto: MZUSP 89295, 4, 45.8–53.7; Minaçu: MZUSP 54091, 9, 59.7–67.1; Aruanã: MZUSP 91160, 3, 30.7–50.9, rio Araguaia. Bahia: MZUSP 90886, 6, 55.9–61.0 Iaçu, rio Paraguassú, rio São Francisco. Minas Gerais: LBP 10294, 20, 70.7–92.8, São Roque de Minas, rio São Francisco. LBP 10394 12, 58.6–76.0, Buritizeiro, rio São Francisco; MZUSP 47352, 3, 31.6–34.2, Manga, rio Japoré. Tetragonopterus carvalhoi : Brazil, Amapá, Laranjal do Jari, rio Jarí: LBP 5376, 30, 30.3–42.7; LBP 5306, 7 paratypes, 36.7–47.1. Tetragonopterus rarus : Suriname: rio Marowjine: MZUSP 99689, 1, 73.7,. Brazil: Pará, Alto Pará do Oeste, rio Amazonas: MZUSP 88029, 1, 86.6. Tetragonopterus sp: Brazil: Amapá: Laranjal do Jarí, rio Jarí: MZUSP 101472, 10 71.4–79.9,; MZUSP 101714, 12, 67.7–84.3, MZUSP 101755, 3, 56.2–66.0;. Pará: MZUSP 99499, 11, 67.1–78.2 Jacarecangá, rio Teles Pires, rio Tapajós. Tocantins: MZUSP 97933, 6, 57.2–61.0, Alta do Tocantins, rio do Sono. Mato Grosso: MZUSP 91950, 18, 46.0–78.1, Paranatinga, rio Xingu. LBP 1585, 6, 49.3–62.5, Barra do Garças, rio das Garças, rio Araguaia; Cocalinho: LBP 8857, 1, 56.1, lagoa da Montaria, rio Araguaia; LBP 7830, 1, 51.3, rio Araguaia; São Féliz do Araguaia: LBP 4011, 3, 58.5–59.4, Lago Morto, rio Araguaia; LBP 3981, 2, 42.2–44.8, Lagoa Fazenda Taboca, rio Araguaia. Goiás: Aragarças, rio Araguaia: LBP 5751, 6, 51.1–55.7; LBP 1629, 2, 58.7–62.5.
TABLE 1. Morphometric data of Tetragonopterus anostomus sp. n. Range includes holotype and 24 paratypes. SD = standard
| Standard length (mm) Percentage of standard length Greatest depth | Holotype Range 45.4 32.5–45.4 45.4 44.7–49.8 | Mean 46.8 | SD 1.57 |
|---|---|---|---|
| Snout to dorsal-fin origin Snout to pectoral-fin origin | 48.2 47.1–53.3 32.0 28.9–34.2 | 52.6 29.4 | 1.2 1.16 |
| Snout to pelvic-fin origin Snout to anal-fin origin Caudal peduncle depth Caudal peduncle length | 51.6 48.7–53.1 67.3 64.0–68.5 10.5 9.3–12.6 8.9 8.5–11.9 | 49.8 65.4 10.8 9.5 | 1.09 1.31 0.81 0.80 |
| Pectoral fin length Pelvic fin length | 26.4 23.2–27.7 21.1 18.5–21.7 | 25.2 19.7 | 1.25 1.08 |
| Dorsal fin length Dorsal fin base Anal fin length Anal fin base | 39.2 34.8–42.2 17.7 15.1–18.0 15.9 15.9–25.8 35.9 32.8–36.3 | 37.6 16.6 23.8 33.8 | 1.57 1.02 0.84 0.98 |
| Eye to dorsal fin origin Dorsal fin origin to caudal fin origin | 46.1 45.2–50.7 50.6 49.5–58.3 | 50.1 56.3 | 1.10 1.52 |
| Head length Head depth Percentage of head length Snout length | 30.6 28.4–31.4 27.2 24.3–28.6 11.8 9.5–14.3 | 29.0 26.0 10.4 | 0.46 0.63 0.69 |
| Maxillary length Horizontal orbital diameter | 49.0 43.8–51.3 50.8 48.6–52.9 | 50.5 50.5 | 1.64 1.06 |
| Least interorbital width | 29.8 27.9–33.6 | 30.8 | 1.46 |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |