Amazotaxia tetratoma (Chassain, 2011), 2023
|
publication ID |
https://doi.org/10.5281/zenodo.10621733 |
|
publication LSID |
lsid:zoobank.org:pub:DE41BD70-2313-4542-BB55-80C245F37B4F |
|
persistent identifier |
https://treatment.plazi.org/id/927F87BC-FFDD-1A4E-FF20-F98DFE68F901 |
|
treatment provided by |
Felipe |
|
scientific name |
Amazotaxia tetratoma (Chassain, 2011) |
| status |
comb. nov. |
Amazotaxia tetratoma (Chassain, 2011) , new combination
Fig. 23–26 View Figures 23–26
Pleisofornax tetratoma Chassain 2011: 118–120 ; Fig. 3–4 View Figures 1–4 .
Type. Not seen. Chassain (2011) noted the male type was collected by Giuglaris in Bélizon, French Guiana. The holotype for P. tetratoma is in the Chassain collection placed in the Muséum national d’Histoire naturelle in Paris France ( MNHN).
Specimens examined. Five male specimens were available: FRENCH GUIANA: “ French Guiana Belizon , +4.25 -52.65; 2015-06-12, JL Giuglaris leg.” (2, JMC) ; “French Guiana Belizon, +4.25 -52.65; 2015-08, JL Giuglaris leg”
(2, JMC); “ FRENCH GUIANA; BELLIZON; x 2016; lg: J.L. GIUGLARIS” / “Collection of the Global; Eucnemid Research Project’ ( Robert L. Otto)” (green framed white label) (1, GERP) .
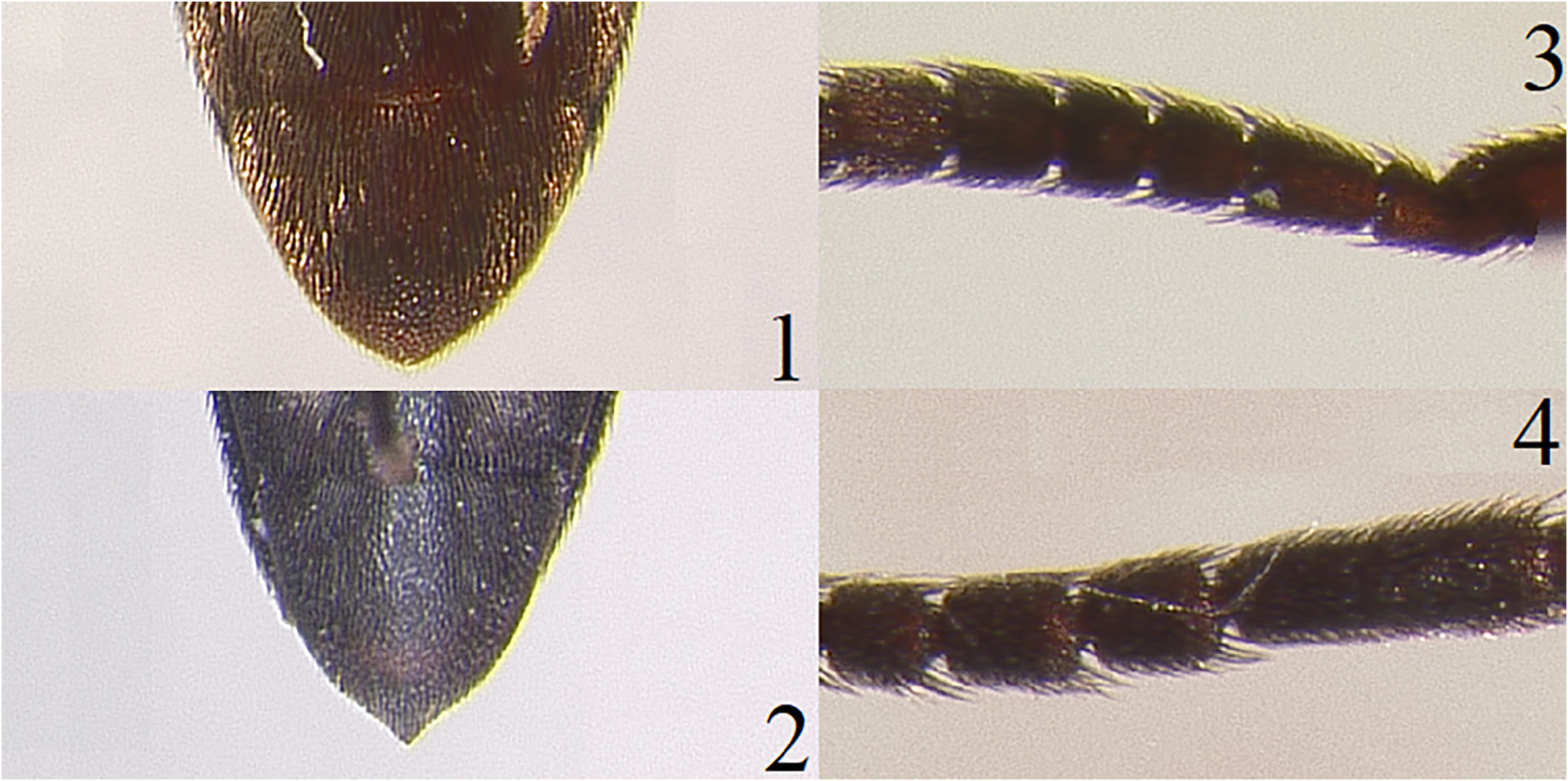
Redescription. Length, 6.0–7.0 mm. Width, 1.75–2.0 mm. Body oblong, elongate; uniformly dark reddish brown; scape, pedicel, and antennal flagellum dark reddish brown; legs, including tarsi, dark reddish brown; head, pronotum, and elytra clothed with short, recumbent, yellowish setae ( Fig. 23 View Figures 23–26 ). Head: Subspherical, without indication of short median carina; surface dullish, rugose; apical margin of frontoclypeal region evenly rounded, about 2 times wider than base; interantennal carina absent at base of frontoclypeal region; mandibles stout, bidentate, densely punctate. Antennae ( Fig. 24 View Figures 23–26 ): Capitate with flagellomeres VI–IX forming an elongate club, reaching nearly 1/2 the length of its body; with last 2 segments extending beyond pronotal hind angles; flagellomere I elongate, almost twice as long as II; flagellomeres II and III subequal, each quadrate and shorter than IV; flagellomeres IV and V subequal, each slightly longer than wide; flagellomeres VI–VIII subequal, each longer than wide and as long as flagellomeres IV–V combined; flagellomere IX slightly longer than VIII. Pronotum: Surface dullish; rugose; slightly longer than wide, with well developed hind angles; lateral sides slightly arcuate, narrowing craniad; disc convex without depressions or carinae; short lineal depression near base, extending 1/4 the length of pronotum above scutellar shield; base sinuous. Scutellar shield: Triangular shaped, longer than wide, slightly rugose, setose, and distally rounded. Elytra: Striae indicated as solid lines; interstices flattened; surfaces dullish, rugose; excretory groove/pit present at elytral apices. Legs: First tarsomere as long as combined length of remaining four on mesothoracic and metathoracic tarsi; tibiae rounded in cross section; lateral surface of mesothoracic and metathoracic tibiae with setae and single spines; metathoracic tarsomeres I–III simple; metathoracic tarsomere IV excavate, as wide as III; metathoracic tarsomere V short; pretarsal claws simple. Venter ( Fig. 25 View Figures 23–26 ): Punctures somewhat deep, very closely dispersed; surface with recumbent, yellowish setae; hypomeron with very shallow, medially undefined lateral antennal grooves; metathoracic episterna parallel sided; elytral epipleura simple, punctate; metathoracic coxal plates medially 3.0–6.0 times wider than laterally; last abdominal ventrite apically rounded.
Sexual dimorphism. Females are unknown.
Aedeagus ( Fig. 26 View Figures 23–26 ). Basal piece longer than wide, laterally parallel sided and narrowing apically, dorsally opened, apically truncated; remaining parts elongate, basal 1/3 narrowed, apical 2/3 wider; lateral sides sinuous; parameres short, apically rounded; lateral tooth present at midway on each paramere; median lobe elongate and basally narrowed, apically pointed, bifid, and much longer than the parameres.
Distribution. Chassain and Touroult (2011) reported this common, endemic eucnemid species from a number of localities in French Guiana.
Biology. The holotype was taken from a flight intercept trap (Chassain 2011). SEAG (Société entomologique Antilles Guyane) has employed a number of trapping techniques through use of Malaise traps and various light traps during their expeditions into the Amazonian rain forests in French Guiana and is possible that all known adult specimens have been taken through these methods. Larvae and pupae are unknown.
Note. The illustration of the genitalia provided by Chassain (2011: figure 4) for P. tetratoma do not match with the observed dissected aedeagus associated with the examined male specimen before me. The illustrated aedeagus showed the presence of secondary lateral lobes basally attached to the lateral lobes. Chassain (2011) made no mention of the presence of secondary lateral lobes in the original description, which questions the inclusion of the illustration for the aedeagus associated with the species. The dissected aedeagus associated with one of the specimens before me lacks these structures, which is problematic for diagnostic purposes. Further research on this issue will be conducted during the forthcoming faunal research for Eucnemidae in French Guiana in the near future.
Diagnosis and discussion. Chassain (2011) wrote P. tetratoma is intermediate between Dyscolotaxia and Plesiofornax . He felt the species is more closely allied to Plesiofornax . However, after observing the external morphology of P. tetratoma , the species does not fit in either Dyscolotaxia or Plesiofornax . A number of character states were discovered to distinguish the new group from Dyscolotaxia and Plesiofornax . Firstly, the absence of transverse spines on the lateral sides of the mesothoracic and metathoracic tibiae and secondly, the slightly wider base of the frontoclypeal region. Dyscolotaxia and Plesiofornax have transverse rows of spines present on the lateral sides of the mesothoracic and metathoracic tibiae and have a narrower base of the frontoclypeal region (especially in Dyscolotaxia ). Amazotaxia have a wider base of the frontoclypeal region and lack transverse rows of spines on the lateral surfaces of the mesothoracic and metathoracic tibiae. Thirdly, the presence of specialized sex patches on abdominal sternites 2 and 3 in male specimens of Dyscolotaxia will further distinguish Dyscolotaxia from both Amazotaxia and Plesiofornax . Lastly, capitate, tubular antennae will further distinguish both Amazotaxia and Dyscolotaxia from Plesiofornax . Plesiofornax have serriform antennae with antennal flagellomeres III–VIII being subequal. These observed character states provide enough evidence to warrant the description of a new genus group.
Amazotaxia appear to be more closely related to Dyscolotaxia based on overall external morphology and restricted Neotropical distribution compared to Plesiofornax . It is impossible to tell if the loss of features such as the medial ridge along the lateral antennal grooves of the hypomeron, along with transverse spines on the lateral sides of the mesothoracic and metathoracic tibiae, constitutes derived features or primitive features retained by the group. Amazotaxia are restricted to the Amazonian rainforest in French Guiana. It is possible the genus may also occur in northern Brazil, adjacent to the borders of French Guiana and Suriname. Further surveys in both countries, along with areas such as Guyana and Venezuela, will help explore the extent of the species’ range in South America.
Dyscolotaxia are seemingly restricted to the extreme northwestern areas of South America, from Ecuador, presumably north through Colombia, to Panama and Costa Rica, and on Hispaniola. Dyscolotaxia have not been reported from Colombia, though it is probable that the genus is present. Dyscolotaxia appear to be restricted to the valleys or basins within the series of Cordilleras or ranges of the Andes Mountains in both Ecuador and Colombia. Further surveys in other parts of South America, Lesser Antilles, Greater Antilles ( Cuba, Puerto Rico, Jamaica), and areas north of Costa Rica may yield additional information on the extent of their range in the Neotropical region.
The presence of D. hispaniolensis new species in Dominican Republic within the Antilles represents an interesting discovery. The new species is undoubtedly a Dyscolotaxia , based on observed external morphology of the new species. The presence of an apically produced last abdominal ventrite along with the loss of the lateral spine on the parameres will distinguish D. hispaniolensis from any known species of Dyscolotaxia . The presence of the new Dyscolotaxia species in the Antilles can only be explained through known palaeobiogeographical hypotheses.
Graham (2003) discussed the formation of the Proto-Greater Antilles in the Pacific Ocean during the Cretaceous Epoch and how these islands began to coalesce throughout geological time. Graham further noted these islands (as part of the Caribbean plate) drifted between the North American and South American landmasses and ended up in the Gulf of Mexico where it presently sits after it collided with the Bahamas Platform. Pindell and Kennan (2009) continued that discussion by modifying the formation hypothesis into the theory that is generally accepted today. They believed the Antilles and Aves Ridges were originally subaerial to a submerged chain of volcanic edifices formed northwest of the South American continent in what is present day Columbia/ Ecuador, which connected the continent to North America during the Cretaceous Epoch (130–110 million years ago). As the plate broke away and drifted northeastward, it collided with the Florida and Bahamas platform and created two deformational belts to the north, allowing Cuba, Hispaniola, Puerto Rico, and the Virgin Islands to uplift. Tobago and the leeward Antilles were also uplifted as the plate collided with the Columbian and Venezuela plates in the south during the Eocene Epoch (57 million years ago). As the Greater and Lesser Antilles uplifted from these collisions, volcanism continued in the Aves Ridges and lasted from 75–48 million years ago, creating the volcanic chain that we see today. Since then, volcanism has ceased in the Aves Ridges ( Aitken et al. 2009).
One of the much-discussed hypotheses on Caribbean biogeography is the GARRLandia hypothesis by Iturralde-Vinent and MacPhee (1999), which suggests that the Aves Ridge emerged from the oceanic surface to create a landmass where it began at northern South America and terminated at Cuba /Hispaniola (Greater Antilles). This landmass existed for 2 million years and allowed organisms to cross into the Caribbean region before the ridges submerged beneath the oceanic surface. Iturralde-Vinent and MacPhee used fossil evidence to explain how some mammals reached the Greater Antilles in the Caribbean. However, their hypothesis generated discussion and controversy regarding how these islands in the Caribbean became populated. That hypothesis was largely dismissed by Ali (2012) and Ali and Hedges (2021) based on phylogenetic evidence of different vertebrates, including reptiles, amphibians, and mammals by examining how various clades colonized these islands in the Caribbean. Iturralde-Vinent and MacPhee believed these islands were populated by vicariance through long distance and organisms were dispersed over water from a nearby continent, especially invertebrates and plants.
Other studies offer ideas of colonization through vicariance, in which organisms colonized these islands after arriving from the continent. Rosen (1975, 1978 and 1985) published his studies discussing his hypothesis of island colonization of megafauna such as reptiles and mammals. He suggested these animals arrived from nearby continents and colonized these islands, which, through endemism by vicarance, independently evolved into new species while the West Indies drifted away from these landmasses.
Roncal et al. (2020) examined four primary hypotheses of island colonization, including vicariance, GAARlandia, long distance dispersal, and in-situ speciation (speciation occurring within or between islands) by using plants from the Caribbean to revise the discussion of regional paleogeography. Roncal et al. used the Time-For- Speciation Effect hypothesis to examine colonization time for groups of plants in the region through phylogenetic analysis. Mathematical equations were used to calculate estimated time of colonization occurring for each group. Their results that showed plant colonization took place repeatedly over the last 60 million years from continental America, especially from Central and South America. Most colonization (over 80%) took place after the purported GARRlandia event as proposed by Iturralde-Vinent and MacPhee (1999), thereby refuting that hypothesis along with the Cretaceous vicariance model from Rosen (1975). Through their models, Roncal et al. concluded that over water dispersal (especially for seed plants) from Central and South America allowed plants to colonize and evolve on these islands. Most colonization occurred more recently than the Oligocene Epoch.
After the closure of the Panamanian Isthmus more than 3 million years ago, biotic exchanges between the North American (higher elevation species) and South American (lower elevation species) landmasses commenced, which allowed Dyscolotaxia (originally a South American group) and other biotic groups to extend their ranges through Central America northward. This may explain why there are no collection records for Dyscolotaxia north of Costa Rica.
| MNHN |
Museum National d'Histoire Naturelle |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Amazotaxia tetratoma (Chassain, 2011)
| Otto, Robert L. 2023 |
tetratoma
| Chassain 2011: 118 - 120 |