Oxya agavisa Tsai, 1931
|
publication ID |
https://doi.org/ 10.5281/zenodo.185158 |
|
DOI |
https://doi.org/10.5281/zenodo.6220013 |
|
persistent identifier |
https://treatment.plazi.org/id/934B87AC-A549-FFE3-FF4F-FF338D4B60B4 |
|
treatment provided by |
Plazi |
|
scientific name |
Oxya agavisa Tsai, 1931 |
| status |
|
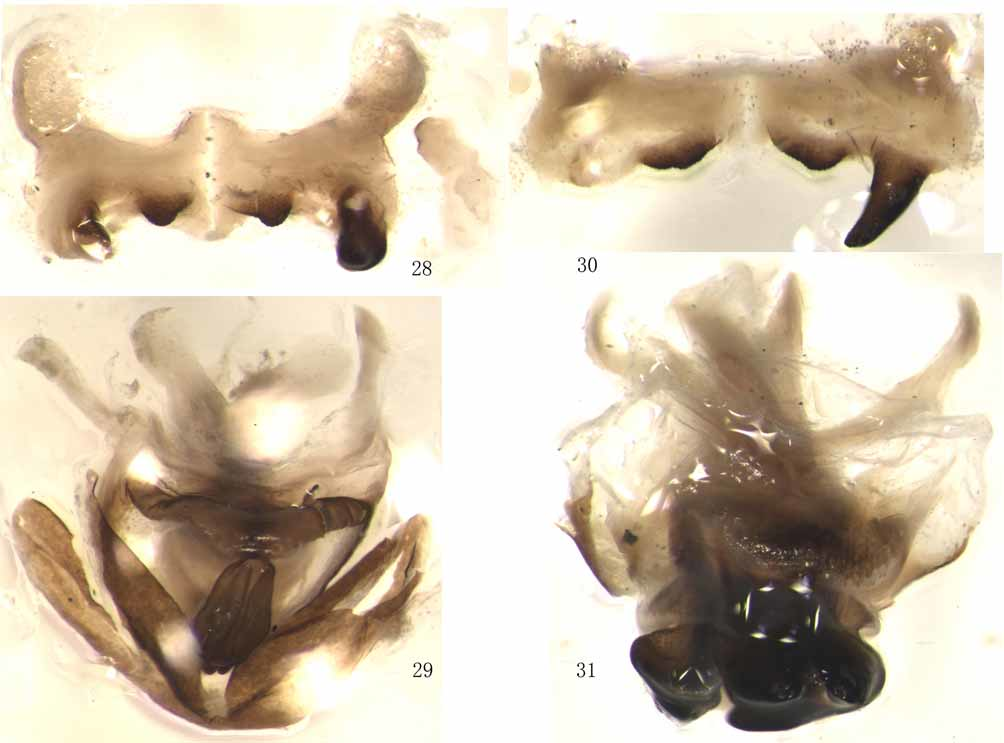
[ Figs. 1–15 View FIGURES 1 – 4. 1 – 2 View FIGURES 5 – 7. 5 View FIGURES 8 – 11 View FIGURES 12 – 15 , 28–29 View FIGURES 28 – 31. 28 – 29 ]
Oxya agavisa Tsai, 1931: 437 ; Chang, 1934: 186; Tinkham, 1940: 296; Bey-Bienko & Mishchenko, 1951: 165; Mishchenko, 1952: 151; Xia, 1958: 34; Hollis, 1975: 222; Zheng, 1985: 129; Storozhenko, 1992: 37; Zheng, 1993: 81; Ma, Guo & Zheng, 1993: 212; Liu, Ding, Long, et al, 1995: 54; Yin, Shi & Yin, 1996: 483; Jiang & Zheng, 1998: 89; Ren, Ma & Guo, 2002: 507; Zhang, Ma & Guo, 2003: 533; Li, Xia, et al, 2006: 84; Yin, Yin & Zheng, 2008: 67.
Oxya agavisa agavisa Tsai ; Hollis, 1971: 317.
Oxya agavisa form robusta Tsai, 1931: 439; Tinkham, 1940: 296.
Oxyoides wulingshanensis Zheng & Fu, 1994: 100 ; Li, Xia, et al, 2006: 88; Huang, Fu & Zhou, 2007: 528. New synonym
Oxyoides wulingshanensis Fu, Peng & Zhu, 1995: 58 .
Male. Body length: 24.4–34.0 mm; pronotum length: 4.1–7.2 mm; Tegmina length: 14.5–27.0 mm; hind femur length: 14.0–17.0 mm.
Body medium-sized. Integument finely punctured and shiny. Face slightly oblique in profile view; frontal ridge shallowly sulcate, lateral sides nearly parallel. Eyes large and oval. Vertex short, fastigium slightly rounded and as long as broad, interocular distance slightly wider than frontal ridge between antennal sockets. Antennae filiform, twenty-five- to twenty-seven-segmented, slightly exceeding posterior margin of pronotum, with median segments two and a third times as long as broad. Pronotum subcylindrical, with dorsum slightly flattened, hardly narrowing forwards, median carina weak, lateral carina absent, posterior margin obtuseangular, three transverse sulci distinct and prozona slightly longer than metazona. Prosternal process conical, with thick base and slightly pointed apex. Mesosternal interspace narrow, three times as long as its minimum width. Tegmina fully developed, not or hardly surpassing apex of hind femora; hind wing as long as tegmina. Hind femora moderately slender, with upper median carinae smooth, both inner and outer lower genicular lobes sharply spined; hind tibiae expanded in apical half and with acute dorsolateral margins, both internal and external apical spines present; hind tarsi with large arolium. Terminal tergite of abdomen without furcula. Supra-anal plate rounded triangular, with weak basilateral folds and a short shallow longitudinal sulcus in the middle portion of the base. Cerci conical, with broad truncate apex. Subgenital plate short conical, with pointed, broadly rounded or distinctly truncate apex. Epiphallus completely divided into two symmetric half, with narrow bridge, a large pair of slender, hook-like outer lophi and a small pair of tooth-like inner lophi, without ancorae; anterior projections large, distinctly exceeding beyond the anterior margin. Phallic complex with posterior process of cingulum, from above, large and rounded trapezoid; lateral fleshy lobes not visible from above; valvular plate of cingulum with a broad, deep posterior emargination, apical valves of penis long, slender, up-curved.
Body color varying, green or brownish green, or dorsal surface yellowish brown and lateral surface green. Postocular bands broad and dark brown. Tegmina brown. Hind femora green or yellowish brown, knee dark brown. Hind tibiae green or bluish green, and dark at base, metatibial spines with apical half black.
Female. Body length: 28.0–39.0 mm; pronotum length: 5.3–8.8 mm; Tegmina length: 18.0–32.0 mm; hind femur length: 17.0–22.0 mm.
Similar to male. Body larger and more robust than male. Antennae slightly shorter than combined length of head and pronotum. Anterior margin of tegmina weakly spined. The second, third and fourth abdominal tergite with posterolateral spines. Valves of ovipositor with tooth-like spines, posterior ventral basivalvular sclerite with a large spine on its inner ventral margin. Ventral surface of subgenital plate with deep median posterior concavity bordered on either side by a strong lateral longitudinal ridge which bears spines along its length; posterior portion of subgenital plate, excluding spines, with a triangular profile, median pair of spines well developed and closely spaced, two small pairs of lateral spines present.
Materials examined. Oxyoides wulingshanensis : one male, holotype (abdominal apex missing), CHINA: Zhangjiajie (present name for former Dayong), Hunan Province, 29°10'N, 110°50'E, 17 August 1990, Guosheng Peng ( Figs. 1–2 View FIGURES 1 – 4. 1 – 2 ). Oxya agavisa : one male with ill-developed tegmina, CHINA: Jinggangshan, Jiangxi Province, 18 October 2001, Zizhong Liao ( Figs. 3–5 View FIGURES 1 – 4. 1 – 2 View FIGURES 5 – 7. 5 ); one male with ill-developed tegmina, CHINA: Liangtouyang Nature Reserve, Fenghuang County, Hunan Province, 30 July 2004, Jianhua Huang ( Figs. 6–7 View FIGURES 5 – 7. 5 ); three males with normal tegmina, CHINA: Hengshan Nature Reserve, Hengshan county, Hunan Province, 28 August 2007, Jianhua Huang ( Figs. 8–15 View FIGURES 8 – 11 View FIGURES 12 – 15 ); in addition, more than fifty individuals from Hunan, Guangxi, and Guangdong Province were examined.
Distribution. CHINA (Shanghai, Jiangsu, Zhejiang, Anhui, Fujian, Jiangxi, Hubei, Hunan, Guangdong, Guangxi, Sichuan, Guizhou, Yunnan).
Remark. Oxyoides wulingshanensis looks very similar to Oxya agavisa except much more reduced tegmina and hind wing as well as truncate apex of subgenital plate in male ( Zheng & Fu, 1994). However, we find that both tegmina and hind wing of Oxyoides wulingshanensis show a slightly burnt appearance, which indicates that the type specimen of Oxyoides wulingshanensis may be a poorly-nourished individual of Oxya agavisa with poorly-developed tegmina and hind wings. Besides, we find two other male individuals of Oxya agavisa with obviously ill-developed tegmina and more or less truncate apex of subgenital plate, showing extreme similarity to the type specimen of Oxyoides wulingshanensis ( Figs. 3–7 View FIGURES 1 – 4. 1 – 2 View FIGURES 5 – 7. 5 ). Furthermore, we also find some male individuals of Oxya agavisa with normal tegmina and more or less truncate apex of subgenital plate ( Figs. 8–15 View FIGURES 8 – 11 View FIGURES 12 – 15 ). Since the external character of “reduced tegmina” and “truncate apex of subgenital plate” that distinguish Oxyoides wulingshanensis from Oxya agavisa either is questionable or can be found in normal individuals of Oxya agavisa , and the individual from Jinggangshan Nature Reserve, with ill-developed tegmina and truncate apex of subgenital plate, extremely similar to Oxyoides wulingshanensis ( Figs. 3–5 View FIGURES 1 – 4. 1 – 2 View FIGURES 5 – 7. 5 ), have no difference from normal individuals of Oxya agavisa in the male genitalia structure ( Figs. 28–29 View FIGURES 28 – 31. 28 – 29 ), we conclude that they are conspecific.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Oxyinae |
|
Genus |
Oxya agavisa Tsai, 1931
| Huang, Jianhua, Zheng, Zhemin, Huang, Yuan & Zhou, Shanyi 2009 |
Oxyoides wulingshanensis
| Fu 1995: 58 |
Oxyoides wulingshanensis
| Huang 2007: 528 |
| Li 2006: 88 |
| Zheng 1994: 100 |
Oxya agavisa agavisa
| Hollis 1971: 317 |
Oxya agavisa
| Yin 2008: 67 |
| Li 2006: 84 |
| Zhang 2003: 533 |
| Ren 2002: 507 |
| Jiang 1998: 89 |
| Yin 1996: 483 |
| Liu 1995: 54 |
| Ma 1993: 212 |
| Storozhenko 1992: 37 |
| Zheng 1985: 129 |
| Hollis 1975: 222 |
| Xia 1958: 34 |
| Mishchenko 1952: 151 |
| Bey-Bienko 1951: 165 |
| Tinkham 1940: 296 |
| Chang 1934: 186 |
| Tsai 1931: 437 |
Oxya agavisa
| Tinkham 1940: 296 |
| Tsai 1931: 439 |