Microthalestris littoralis Sars, 1911
|
publication ID |
https://doi.org/10.11646/zootaxa.5051.1.13 |
|
publication LSID |
lsid:zoobank.org:pub:F94203E7-FCD1-4975-BAD3-0DF534806712 |
|
DOI |
https://doi.org/10.5281/zenodo.5572446 |
|
persistent identifier |
https://treatment.plazi.org/id/951887EA-FFE6-FFB9-FF51-D147E149FE76 |
|
treatment provided by |
Plazi |
|
scientific name |
Microthalestris littoralis Sars, 1911 |
| status |
|
Microthalestris littoralis Sars, 1911
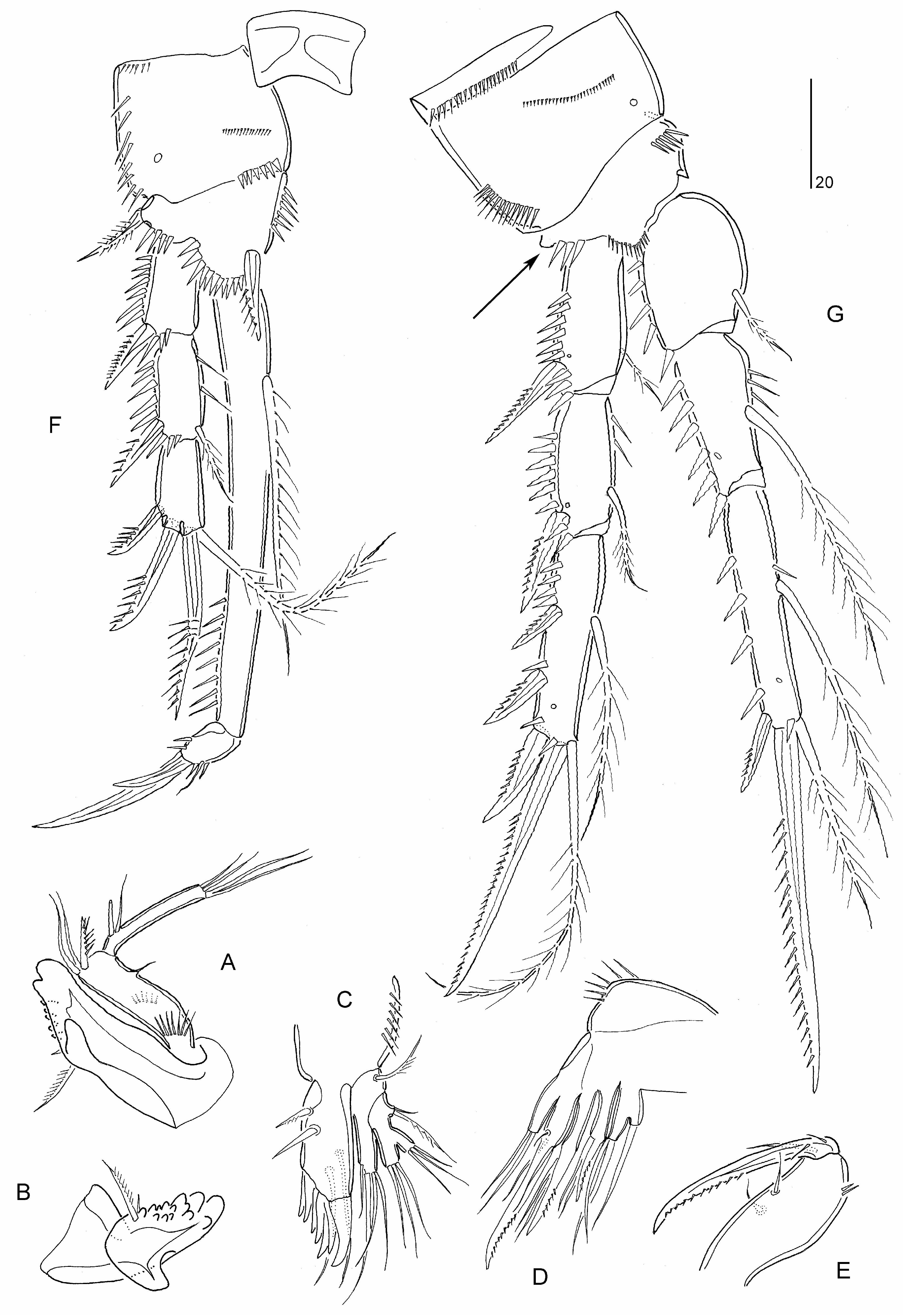
Comparison of the various reports dealing with M. littoralis reveals the true state of confusion reigning in the genus. In Norway the species shows virtually the same distribution as M. sarsi sp. nov., extending from the south coast northwards to the Trondhjem Fjord ( Sars 1911). Several authors have reported on the sympatric occurrence of M. littoralis with other species but in the great majority of the cases the authenticity of the former is rendered doubtful by the absence of an adequate description or a definite statement of authority for the identification. For example, according to Wells (1961) three forms of P. spinosa ( typica , littoralis, bulbosa ) occur sympatrically in the Isles of Scilly while the latter two also coexist in the River Exe estuary ( Wells 1963a). Both Monard (1935a) and Jakubisiak (1936) found that M. littoralis and M. forficula are sympatric in the Roscoff region in Brittany while Wilson (1932b) recorded both species from the Woods Hole region. Farran (1913) documented significant variation in female body size (500–850 μm) in his material of M. littoralis from Clare Island and Blacksod Bay in Ireland which he perceived as a possible indication of the presence of a second species. Lang’s (1934, 1936b, 1948) failure to recognize the sympatric occurrence of Microthalestris species and his subsequent proposal of an all-encompassing, highly variable species concept of P. spinosa are the primary reasons why most published records of both M. littoralis and M. sarsi sp. nov. cannot be accepted as accurate. Although most workers accepted Lang’s views and perpetuated the myth of a cosmopolitan morphologically plastic species, others advocated that M. littoralis deserved distinct species status (e.g. Willey 1935; Jakubisiak 1936; Sewell 1940; Nicholls 1945; Roe 1958, 1960; Pór 1960). Sars (1911) claimed that M. littoralis differed from his M. forficula (= M. sarsi sp. nov.) in the more compact antennule, the longer and more slender P1, the number of exopodal setae on the P5 (six vs eight) and the normally developed seta V on the caudal rami. Although the species was recorded from virtually all around the Norwegian coastline, no males were discovered by Sars (1911). Despite the absence of information on the armature formula of P2–P4, M. littoralis is reinstated here based on the differences listed above. The only reliable records of the species are those by Sars (1911).
Several authors have provided concise illustrated descriptions of what they believed to be M. littoralis . Brian’s (1921: 77–80) description of M. littoralis from the Gulf of Genoa included detailed illustrations of various developmental instars (naupliar stages I, III–V and copepodids III–V) while his descriptions of the adults are unfortunately not of a comparable standard. Little useful information is revealed about the morphology of the female except for the presence of a single egg sac and the normally developed caudal ramus seta V. The armature formula of P1–P5 is completely unknown although Brian (1921: 78) does mention that the distal endopodal segment of P3 has one spine and three plumose setae (“… una gracile spina e tre setole piumate…”). His description of the male shows the typical sexual dimorphism on the P3 endopod (bearing an inner seta on enp-1 and -2, and an apophysis and two apical setae on enp-3), and the 1-segmented condition of the male P5 exopod (bearing seven setae). The presence of two penicillate spines on the antenna (already expressed in copepodid IV: his Plate VIII, Fig. 17) provides evidence that Brian’s (1921) material does not belong to Microthalestris , representing instead a member of Penicillicaris gen. nov. (see below). Given its geographical proximity it may be conspecific with Car’s (1884) Thalestris pectinimana .
Wilson (1932b: 205) recorded ten females from Cuttyhunk Harbor, Massahusetts which he identified as M. littoralis . Unfortunately, the only drawings of the habitus in dorsal aspect and the P5 are adapted from Sars (1911), making it impossible to decide on the identity of his material. Lang’s (1934) illustrated record of P. forficula var. littoralis from Campbell Island differs from M. littoralis in the morphology of the P1, female P5 and caudal ramus seta V and is here renamed as M. campbelliensis sp. nov. (see below).
Willey (1935: 82–83) recorded one ovigerous female from Harrington Sound in Bermuda which according to the author agreed with Sars’s (1911) description of M. littoralis in displaying 9-segmented antennules (although the segmentation was not disclosed by Sars) and unmodified caudal ramus setae. Much of Willey’s discussion centres around morphological differences in the antenna and swimming legs encountered between this “typical” specimen and his more abundant, sympatric, forma penicillata . The latter is here upgraded to species level and fixed as the type of Penicillicaris gen. nov. (see below) while the former is considered indeterminable based on the limited information presented by Willey.
Monard’s (1937: 59, 62, Fig. 3–E View FIGURE 3 ) description of P. forficula littoralis from Algeria, presenting a single illustration of the male P5 exopod, does not contain the bare minimum required for any meaningful statement on its identity.
Sewell (1940: 191–196) distinguished three different forms in his material of Parastenhelia littoralis obtained from seaweed washings in Addu Atoll, Maldives. The typical form, which he considered identical with Sars’s (1911) M. littoralis , shows distinct differences with the original description in the female antennule, P1 and P5, unambiguously ruling out such conspecificity. The presence of penicillate spines on the antennary endopod and on exp-3 and enp-2 of P1 indicates that Sewell’s (1940) material represents a member of Penicillicaris gen. nov. (see below). Given their morphological disparity, we cannot concur with the author that both Thalestris forficula sensu Thomson (1883) and M. forficula sensu Monard (1928) are misidentified records of M. littoralis .
Nicholls (1945: 4) provided illustrations of both sexes of Parastenhelia forficula var. littoralis from Port Denison in Western Australia. Females differ slightly from Sars’s (1911) description of M. littoralis in (a) the proportionally longer P1 exp-2 and enp-1, and (b) the P5 endopodal lobe which is narrower and less truncate. Pending information on the antennule, antenna and swimming legs in both forms and on the male of M. littoralis , the Australian population is provisionally regarded as distinct from the Norwegian one.
Original description. Sars (1911): 369–370, Supplement plate 11, Fig. 1 View FIGURE 1 .
Type locality. Sars (1905) recorded material from several places on the south and west coasts of Norway, and in the Trondhjem Fjord. Since he did not specify which specimens the illustrations were based on, the type locality encompasses all of their respective places of origin ( ICZN Art. 73.2.3) .
Differential diagnosis. Microthalestris . Body length 600 μm in ♀, unknown for ♂. Antennule segmentation and antenna unconfirmed. P1 exopod about 80% length of endopod; exp-2 elongate, about twice as long as exp-1, and about half as long as enp-1; insertion point of inner seta of enp-1 at 25% of inner margin length; exp-3 with two unipinnate spines and two geniculate setae; enp-2 with one minute seta, one geniculate seta and one geniculate claw. Armature pattern of ♀ P2–P4 and ♂ P3 endopod unknown. P 5 ♀ with elongate exopod (about three times as long as maximum width), inner margin and proximal two-thirds of outer margin straight, with six elements; endopodal lobe with five elements, innermost one short. P5–P 6 ♂ unconfirmed. Caudal ramus seta V normally developed .
Notes. Most records of M. littoralis (either as a distinct species or as a form of Parastenhelia forficula or P. spinosa ) are not accompanied by a description and must therefore remain indeterminable:
Sweden: Øresund ( Lang 1936b – as Parastenhelia forficula var. littoralis ).
Ireland: Clew Bay and Blacksod Bay, Co. Mayo ( Farran 1913; Farran et al. 1915); Clare Island ( Southern 1915); Dalkey Island and The Muglins, Co. Dublin ( Roe 1958 – as Parastenhelia spinosa var. littoralis ); Lough Hyne (Ine), Co. Cork ( Roe 1960 – as P. spinosa var. littoralis ).
Northern Ireland: Ardglass and Kilclief, Co. Down ( Williams 1954).
England: Isles of Scilly ( Wells 1961 – as P. spinosa forma littoralis ); River Exe ( Wells 1963a – as P. spinosa forma littoralis ).
France: Roscoff, Brittany ( Monard 1935a; Jakubisiak 1936).
Italy: Gulf of Genoa Brian (1917, 1921); Sardinia ( Brian 1923a).
Croatia: Palagruža ( Steuer (1912); Rovinj ( Brian 1923b; Vàtova 1928); Island of Šolta ( Jakubisiak 1933).
Greece: Dodecanese Islands ( Brian 1928a); Rhodes, Astypalaia, Tilos, Symi, Kos, Karpathos ( Brian 1928b).
Algeria: Îlot de la Marine (Amirauté) and Bab-el-Oued ( Monard 1936 – as Parastenhelia (Microthalestris) forficula littoralis ); Algiers and Bou Ismaïl (Castiglione) ( Monard 1937 – as P. forficula littoralis ).
Ukraine: Yalta, Crimean Peninsula ( Marcus & Pór 1960 – as Parastenhelia littoralis ).
Bermuda: Harrington Sound ( Willey 1935).
U.S.A.: Chesapeake Bay ( Wilson (1932a); Cuttyhunk Island ( Wilson (1932b).
India: Porites Bay off Krusadai Island, Tamil Nadu ( Krishnaswamy 1957); Visakhapatnam coast ( Sarma 1974 a, 1974b, 1974c; Krishna Murty 1983) and Visakhapatnam Harbour ( Sarma & Ganapati 1975) in Andhra Pradesh (all as Parastenhelia littoralis ).
Andaman and Nicobar Islands: South Andaman ( Jayabarathi 2016 – as Parastenhelia spinosa littoralis ) .
Malaysia: Peninsular Malaysia ( Zaleha et al. 2010 – as Parastenhelia littoralis ); Straits of Johor ( Mahadi et al. 2014).
Australia: Port Denison, Western Australia ( Nicholls 1945 – as Parastenhelia forficula littoralis ).
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |