Iberis umbellata, (L.)
|
publication ID |
https://doi.org/10.1016/j.phytochem.2018.12.010 |
|
DOI |
https://doi.org/10.5281/zenodo.10576607 |
|
persistent identifier |
https://treatment.plazi.org/id/97758935-FFE3-FFDC-A678-FF20079DCF52 |
|
treatment provided by |
Felipe |
|
scientific name |
Iberis umbellata |
| status |
|
2.1. Anthocyanins in the red-purple flowers of I. umbellata View in CoL
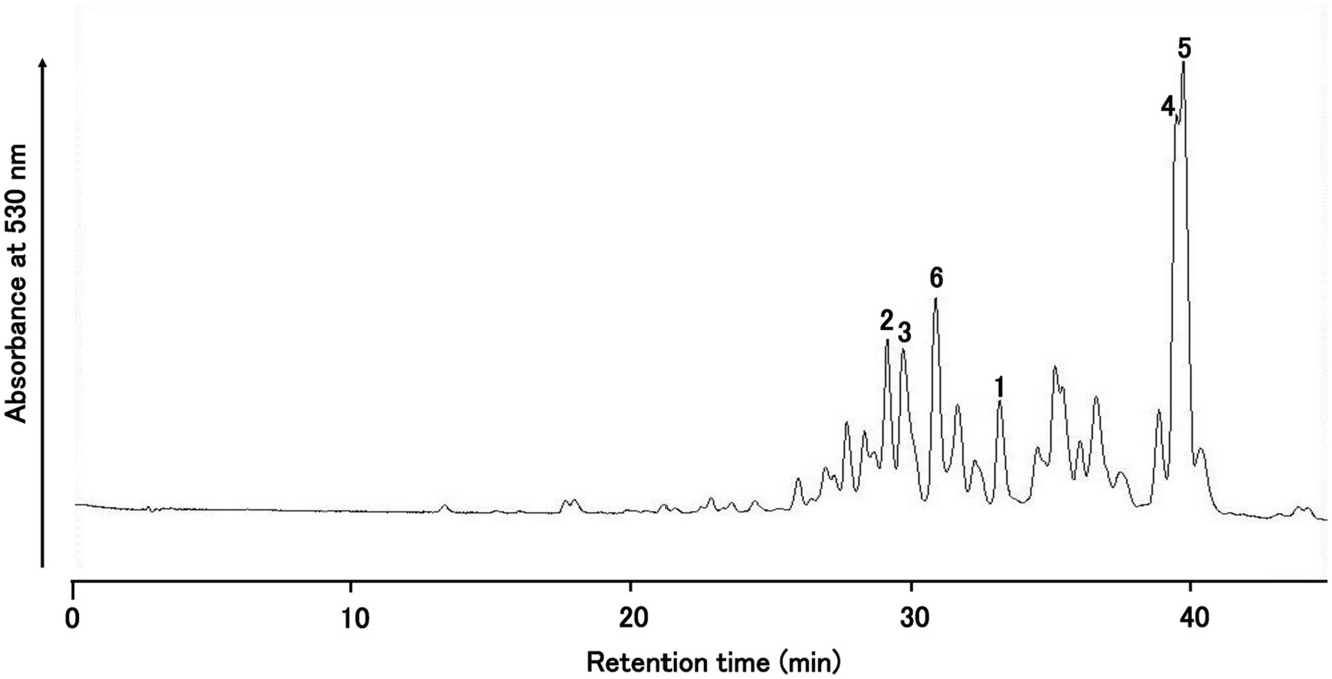
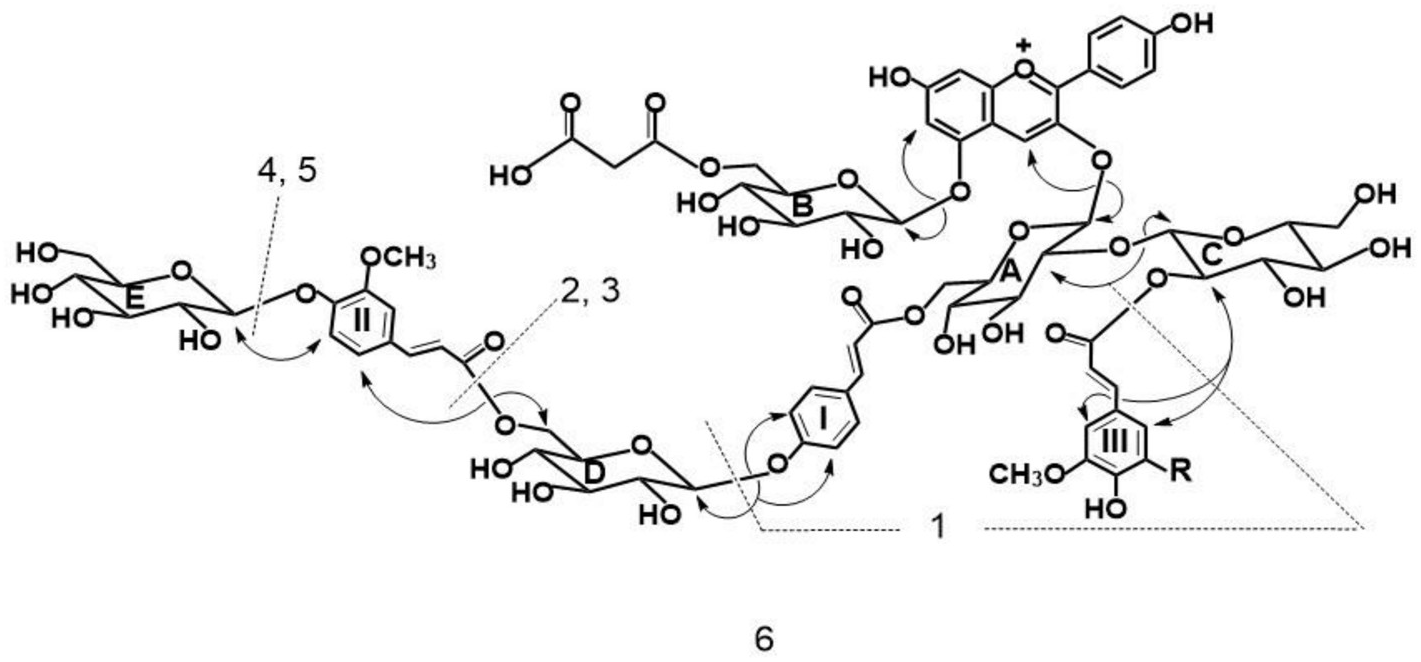
By HPLC analysis of an extract from the red-purple flowers of Iberis umbellata , I identified more than 20 anthocyanin peaks. Among these peaks, six major anthocyanin peaks were isolated from the flowers of ‘Candycane Red’ ( Fig. 1 View Fig ) and ‘Candycane Rose’ by extraction by 5% HOAc, and purified according to the procedure described previously ( Saito et al., 2008). The same six pigments were found in both cultivars, but the profiles differed slightly. Percentages of total anthocyanin contents based on HPLC peak aerias at 530 nm are as follows for the two cultivars: ‘Candycane Red’: 1: 4.0%, 2: 5.8%, 3: 8.0%, 4: 10.3%, 5: 15.9%, 6: 7.6%; ‘Candycane Rose’ 1: 5.6%, 2: 13.4%, 3: 6.9%, 4: 9.6%, 5: 6.9%, 6: 8.3%. The chromatographic and spectroscopic properties of these pigments are summarized in Experimental. Visible maxima ( λ max) from UV–VIS spectra of 1–6 in 0.1% HCl-MeOH were observed at 508–519 nm. In addition, the ratio of absorbance at 440 nm relative to the visible maximum ( E 440 / E vis.max) were 0.17–0.20. With the addition of AlCl 3, 1–6 did not exhibit a bathochromic shift, indicating that they lacked a vicinal hydroxyl group in the B-ring. Therefore, these initial data suggest that 1–6 may have a pelargonidin 3,5-glycosyl type structure ( Tatsuzawa and Shinoda, 2005). This was subsequently confirmed by the NMR analysis. The ratio of E acyl / E vis.max was 0.90–1.63 suggesting that they had one, two or three hydroxycinnamic acid units ( Saito et al., 2008) which was also confirmed in the subsequent NMR analysis. Pigment 1 was identified by HR-FAB MS (calc. for C 45 H 49 O 25 requires: 989.2563. Found: 989.2556) and comparison of HPLC, TLC, and UV–Vis data (see section 4.3.1) with pelargonidin 3- O -[2- O -(β-glucopyranosyl)-6- O -( trans -p -coumaroyl)-β-glucopyranoside]-5- O -[6- O -(malonyl)-β-glucopyranoside] obtained from Raphanus sativus ( Tatsuzawa et al., 2008b) ( Fig. 2 View Fig , Fig. S1 View Fig ).
From all pigments 2–6 ( ca. 1 mg each), the same deacyl anthocyanin was obtained from the alkaline hydrolysis (see section 4.4.). These were identified to be pelargonidin 3-sophoroside-5-glucoside by direct comparison of HPLC behavior with those of authentic samples obtained from Raphanus sativus ( Tatsuzawa et al., 2008b) . 4- O -glucosyl-ferulic acid, 4- O -glucosyl- p -coumaric acid, and ferulic acid ( Saito et al., 2008), 4- O -glucosyl- p -coumaric acid and ferulic acid, 4- O -glucosyl- p -coumaric acid and sinapic acid, and 4- O -glucosyl- p -coumaric acid, ferulic acid, and sinapic acid were obtained from the alkaline hydrolysate of pigment 6, pigments 3 and 5, pigment 2, and pigment 4, respectively, and they were identified by comparison of HPLC behavior with those of authentic samples obtained from a purple-violet cultivar of I. umbellata using the same process as for alkaline hydrolysis ( Saito et al., 2008).
Elemental components of 2–6 were confirmed by measuring their high resolution FAB mass spectra (HR-FAB MS) (LMS-700, JEOL Ltd.). To obtain information on the linkages among the aglycone, sugars, and/ or acids as well as to investigate the structural similarity of these pigments, partial hydrolysis (see section 4.5.) of 1–6 was carried with a procedure described previously ( Saito et al., 2008). Pelargonidin 3-sophoroside-5-glucoside was detected in the hydrolysates as intermediary pigment products. The intermediate was confirmed by comparing HPLC results to those of authentic deacylated anthocyanin obtained from Raphanus sativus ( Tatsuzawa et al., 2008b) . Pigment 1 was in the hydrolysate as one of the intermediary pigment products from pigments 2–6. The pigments 1, 3, and 5 were detected in the hydrolysate of 6 as intermediary pigment products. Similarly, 1 and 3 were detected in the hydrolysate of 5. Pigments 1 and 2 were detected in the hydrolysate of 4. Moreover, pigment 1 was detected in the hydrolysate of 2 and 3. From these results, the structures of 2–6 were presumed to be based on pigment 1. The structures of 2–6 were elucidated further based on analyses of their 1 H (400 MHz), 13 C (100 MHz) and 2D (COSY, NOESY, 1 H- 13 C HMQC, and 1 H- 13 C HMBC) NMR (JNM AL-400, JEOL Ltd., Tokyo, Japan) spectra in CF 3 COOD-DMSO‑ d 6 (1:9) with TMS as an internal standard.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |