Abscondita chinensis
|
publication ID |
https://doi.org/10.11646/zootaxa.3721.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:C25F8F57-3875-4E0D-8F34-9DC9C9F876D1 |
|
DOI |
https://doi.org/10.5281/zenodo.6156238 |
|
persistent identifier |
https://treatment.plazi.org/id/9B748785-1273-9401-FF0C-F99E995AD984 |
|
treatment provided by |
Plazi |
|
scientific name |
Abscondita chinensis |
| status |
|
Abscondita chinensis View in CoL (L.) comb. nov.
Figs 6 View FIGURE 6 A; 7–17
Lampyris chinensis L., 1767:645.
Luciola chinensis (L.). Laporte, 1833:149. Lacordaire, 1857:337. Gorham, 1880:101. Olivier, 1885:359; 1902:87; 1912: 89. Bourgeois, 1909:431. Vogel, 1921:269. Liu, 1931:256. McDermott, 1962:27; 1966:101.
Lampyris vespertina Fabricius, 1801:103 .
Luciola vespertina (F.). Motschulsky, 1854:49. Lacordaire, 1857:337. Gorham, 1880:100. Pic, 1932:87.
Luciola praeusta Kiesenwetter, 1874:263 . McDermott, 1966:102. Ganguly, 1980:1. Chen, 2003:176. New synonymy.
Luciola affinis Gorham, 1880:101 . McDermott, 1966:111 (synonymy). Jeng et al. 2003:258.
Luciola gorhami Ritsema, 1883:4 . Gorham, 1883:410; 1895:306; 1903: 327. Olivier, 1902:79; 1907:52; 1912:90; 1913:270. Fletcher, 1919:28. Okada, 1931:145. Miwa, 1931:102. Mehta, 1932:101. Ganguly, 1963: 107. Saxena, 1953a:125; 1953b:197; 1957:71. Lai et al., 1998:210. Jeng et al. 1999:77. Chen, 1999:142. Ho & Su, 2000:54. Nec Luciola praeusta Kiesenwetter. Barua et al., 2007:287 (identification by Ballantyne).
Types. Lampyris chinensis L. not located in Linnaean collection in London by Ballantyne in 1993.
Luciola praeusta Kiesenwetter not located in Munich.
Luciola affinis Gorham not located in MNHN (Jeng examination).
Neotype. Male. CHINA: 31.20N, 114.25E Hubei Province, Huangpi district, Wuhan City, Mt Sushansi, XH Fu (NHMHAU).
Diagnosis. Males and females distinguished from Abs. cerata (Olivier) by their dorsal colouration of pale elytra with black apices ( cerata has black elytra). Most similar to Abs. terminalis (Olivier) with which it shares black terminal abdominal tergites, distinguished by: the ventral abdominal colour ( Abs. chinensis always has V5 completely dark coloured, while in many Abs. terminalis V5 is pale in the middle); the aedeagal sheath sternite terminated by apically acute and divergent lobes (those of Abs. terminalis are rounded and elongate). Differing light patterns are discussed below. Females coloured as for male, differing most obviously from females of Abs. terminalis by the ventral abdominal colour pattern which is either reduced to posterolateral dark patches on V5, or may be absent. Larvae very similar to those of Abs. terminalis , with pale paired anterolateral areas on protergum. Single modulated ca 1 sec male display flash with ca 2.8 sec interval, usually occuring in flight in open forests.
Specimens examined ( Fig. 8 View FIGURE 8 ). Collector is X H Fu. CHINA: N 31.62, E 113.77 Hubei Province, Guangshui District, Suizhou City, 12.vii.2007 9 males. N 31.20, E 114.25 Hubei Province, Huangpi district, Wuhan City, Mt Sushansi, 19.vii.2012 4 males, female; 26.vii.2012 64 males, 28 females. N 31.16, E 115.78, Hubei Province, Tiantang Zhai, Mountain Dabie, 06.vii.2009 12 males 5 females; 13.vii.2010 2 males. N 30.76, E 115.32, Hubei Province, Luotian county, Huanggang City, 02.vii.2006 25 males, 12 females; 28.vii.2006 49 males, 29 females; 23.vii.2007 12 males, 14 females. N 30.39, E 111.45, Hubei Province, Yichang Yidu City, 13.vii.2007 male. N 30.28, E 103.14, Sichuan Province, Mountain Tiantai, 28.vii.2010, 15 males, 3 females. N 30.21, E 114.939 Hubei province, Mountain Dongfang, Huangshi City, 14.vii.2011 9males, 22 females. N 30.18, E 119.24, Zhejiang Province, Mountain Tianmu, 08.vii.2008 14 males; 15.vii.2008 3 males, 10 females; 18.vii.2008 8 males, 1 female. N 22.708, E. 111.994, Hunan Province, Loudi City, 12.vii. 2010, 1 male, 3 females. Additional specimens examined (Jeng et al., 2003): Taiwan: Taipei Co., Hwangshi, 22. Vii. 1996, 3 males, 1 females, M. F. Chen. Taipei, Yentzuhu, 2. Vii. 1996, 2 males, J. Lai. Kaohsiung Co., Jiashan, 4. V. 1997, 1 female, S. K. Chen. Kaohsiung Co., Jiashan, 4. V. 1997, 1 male, S. K. Chen. Pingtung Co., Mancho, 28. iii. 1998, 1 male, 2 femels, J. Lai. Pingtung Co., Kenting, 10. X. 1998, 1 male, 2 females, M.L. Jeng. Pingtung Co., near Jiopong, iii. 1999, 3 males, 1 female, M. L. Jeng. Pingtung Co., Shihai, 19. vii. 2000, 2 males, 2 females, M. L. Jeng. Pingtung Co., Shihai, 19. Vii. 2000, 2 males, 2 females, M. L. Jeng. Pingtung Co., Nanjenshan, 10. Viii. 2000, 3 males, 3 females, M. L. Jeng. Taitung Co., Chenggong, 20. Viii. 2000, 1 female, M. L. Jeng. Taitung Co., Jiben, 20. Iv. 1998, 4 males, 1 female, M. L. Jeng.
Male. Variability in dimensions of different populations shown in Table 5 View TABLE 5 . 5.7–8.3 mm long; 2.0– 2.8 mm wide; approx. 2.5 times as long as wide. Colour: Pronotum orange yellow except for narrow median sulcus sometimes appearing reddish yellow; MS and semi-transparent elytra orange yellow, (except for apical dark brown area occupying 1/17 or less length of elytron) and paler suture which may have underlying fat body along most of its length; MN paler yellow with underlying fat body contributing to colour; head between eyes, antennae and palpi dark brown; ventral thorax orange yellow; all legs orange yellow except for brown tips of femora and dark brown ASW GHW ASD SIW Antennae Body Pronotal Pronotal Elytron Body Specimen length length length width length width information tibiae and tarsi; abdomen orange yellow with ventral brown marking patterns as follows ( Fig. 9 View FIGURE 9 A- J) (number of males in brackets): 1. ( V5 completely dark brown, remaining ventrites yellow) Guangshui Dist, (1); Tiantang Zhai, (2); Luotian county (19), Mt Tiantai, (3); Mt Tianmu (4); Wangjia village (4); Mt Sushansi (6). 2. ( V5 completely dark brown, V4 with small lateral brown spots) Tiantang Zhai (8); Mt Tiantai (15). 3. ( V5 completely dark brown, broader dark lateral areas in V4 not reaching to lateral margins) Guangshui dist. (8); Mt Sushansi (62). 4. ( V5 completely dark brown, dark lateral markings in V4 occupying posterolateral corners) Mt Tianmu (14). 5. ( V5 completely dark brown, posterolateral dark markings in V4 occupying posterior half of V4 and separated in middle by less than their width) Tiantang Zhai, (3); Luotian county (65); Mt Dongfang (8). 6. ( V5 completely dark brown, lateral dark markings in V4 occupying all of the lateral areas and separated in middle by less than their width, with inner margins rounded) Luodi City (1). 7 Wanjia village (1). 8. ( V5 completely dark brown, posterolateral dark areas in V4 separated by their width, in V3 separated by> their width) Mt Tianmu (7). 9. ( V5 completely dark brown, V4 with posterolateral dark markings as for pattern 5, V3 with two posterolateral spots on each side separate from lateral and posterior margins, V3 with single set of spots) Tiatang Zhai (1). 10. ( V5 completely dark brown, posterolateral dark markings on V2, 3, 4 all separated by moe than their width) Luotian county, (2); Mt Dongfang (1). LO in V6, 7 very white, with very narrow yellow lateral margins of V6, and lateral and posterior margins of V7 if visible pale yellow; abdominal tergites 6–8 black, as are dorsally reflexed lateral and posterior margins of V6, and lateral margins of V7; anterior half of dorsally reflexed margins of V5 pale, remainder black; Tergite colouration patterns as follows: 1. (T4 with diffuse median dark markings): Luotian County 29/86; Mt Dongfang 3/9; Tiantangzhai 3/14; Suizhou City 4/9;Mt Tianmu 4/25; Mt Tiantai 9/15; 2. (T4 with dark markings extending across most of the surface and diffuse median dark markings in T3): Luotian County 57/86; Mt Dongfang 6/9; Tiantangzhai 11/14; Suizhou City 5/9’Wangjia Village 5/5; Loudi City 1/1; Mt tianmu 21/25; Mt Tiantai 6/15; Dongshi Forest 1/1.
Pronotum: 1.15–1.6 mm long; 1.8–2.6 mm wide; W/L 1.4–1.8. Elytron: 5.3–6.8mm long. Head: GHW 1.2– 2.15; SIW 0.25–0.4; ASD> ASW. Mouthparts: apical labial palpomere with 3 teeth along inner margins in every population examined. Aedeagal sheath with sternite terminated by narrowing divergent lobes ( Fig. 10 View FIGURE 10 A, B).
Female ( Fig. 7 View FIGURE 7 C, D; 11). 7.2–9.1 mm long; 2.0– 3.1 mm wide; (variation shown in Table 6 View TABLE 6 ); macropterous and observed in flight. Colour: as for male with these exceptions—ventrites anterior to LO yellow except for posterolateral brown markings on V5 (single female from Mt Dongfang with no dark abdominal markings); white LO confined to V6, V7, 8 yellow as are all abdominal tergites. Pronotum: 1.2–1.7 mm long, 2.0–3.0 mm wide. Elytron: 6.0– 7.5 mm long. Head; GHW 1.4–2.1 mm; SIW 0.4–0.55 mm; ASD> ASW; antennae almost always slightly longer than 2 x GHW. Mouthparts: apical labial palpomere with three teeth along inner margins in every population examined. Abdomen orange yellow with ventral brown markings as follows ( Fig. 9 View FIGURE 9 K, L) (number of females in brackets): 1.Luotian county, (55); Mt Dongfang (22); Tiantang Zhai (5); Yidu county, (1); Loudi City, (3); Mt Tiantai (3); Mt Jiugong (2); Mt Zijin (2); Mt Luojia (2); Mt Shusansi (30). 2. Mt Dongfang (1); Mt Tianmu (11); Dongshi Forest (2). Tergite colouration patterns as follows: 1. (T7, 8 pale, T6 dingy at sides and dorsally reflexed margins of V6 very dark) Yidu County 1/1; Loudi City 1/3; Mt Tianmu 3/11; Luotian county 24/55; Mt Dongfang 14/22; Tiantangzhai 1/5. 2. (As for pattern 1 with T5 dingy brown and dorsally reflexed margins of V6 pale) Loudi City 2/3;Mt Tianmu 8/11; Luotian County 29/55; Mt Dongfang 8/22; Tiantangzhai 3/5. 3. (As for pattern 2 with T7 having dark markings at sides and T4 dingy brown in median area) Luotian County 2/55; Tiantangzhai 1/5.
Larva ( Fig. 7 View FIGURE 7 E, F; 12). With pale paired areas on the anterolateral areas of the protergum; similar to larva of Abs. terminalis and not distinguished from it.
Biology: Intermittently glowing larvae, active on the moist soils of the forest, preyed or scavenged on ants and other small insects. Captive larvae fed on fresh-killed ants Polyrhachis vicina Roger ( Fig. 13 View FIGURE 13 A), mealworms Tenebrio molitor and cannibalized when food was scarce. The larvae had five instars, and matured in less than 5 months in lab ( Table 7 View TABLE 7 ) though they had only one generation a year in the wild. Mature larvae constructed pupal cells in soil ( Fig. 13 View FIGURE 13 B) with mean pupal period 7.67±1.72 days (n=15). Pupae were observed (after 3 minutes of dark adaption) to emit a continuous pale luminescent glow from the entire body except for the darkened compound eyes and hind wings ( Fig. 14 View FIGURE 14 A, B, C). The bodies of newly-emergent adults were also observed weakly glowing ( Fig. 14 View FIGURE 14 D), though this luminescence gradually disappeared after c. 3 hours when sclerotization was complete. Mated females laid eggs (n=142) individually which matured in 25.55±1.44 days.
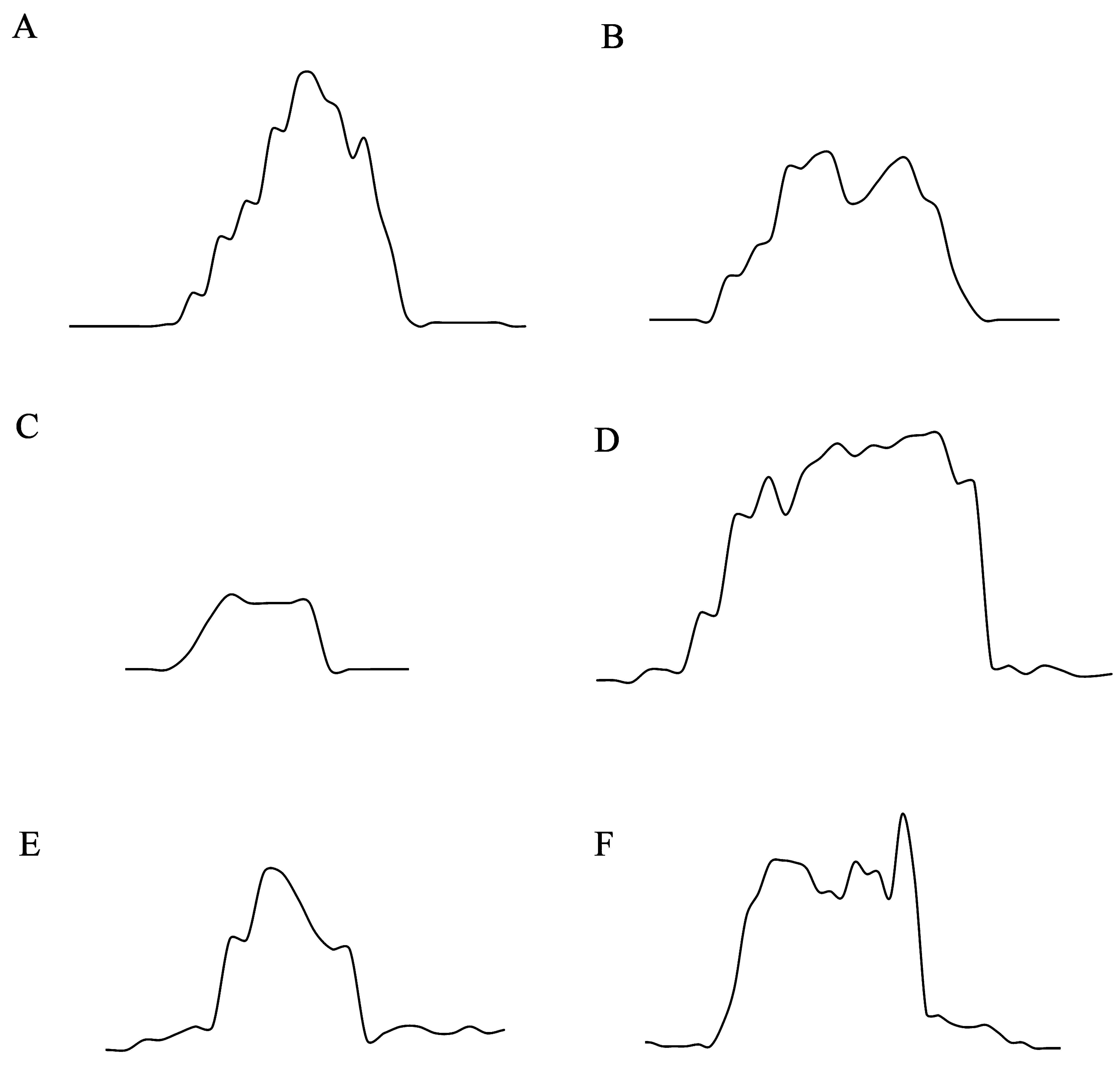
Adult mating season ranged from July to August in Mt. Dabie, Hubei Province. In July, first firefly flash was observed 26 min after sunset; first male flew and flashed 29 min after sunset. The flash activity usually lasted 2 hours with peak at about 1.5 hours after sunset. When patrolling, males usually flew about 2 metres high through the forest and displayed relatively prolonged single-pulse intensity-modulated flashes when searching for females ( Figs. 15 View FIGURE 15 , 16 View FIGURE 16. A ). Under mean temperature 23°C and humidity 89%, flash duration of flying flashing males was 924.39 ± 322.03 ms, interval 2756.54 ± 412.79 ms, and rate 0.28 ± 0.04 (flashes/ sec) (n=19) ( Figs. 15 View FIGURE 15 , 16 View FIGURE 16. A ). Females, walking along the tips of grass and low vegetation on the forest floor, signalled with rapid single pulse flashes with flash duration 201.02 ± 22.02 ms, flash interval 123.97 ± 16.15 ms and rate 3.17 ± 0.55 (n=15). The bioluminescence emission of both the sexes is yellow (λmax= 565nm) ( Fig. 17 View FIGURE 17 ).
*= (Mean ± SD).
Neotype designation. Following article 75.1 of the ICZN, we believe that as no name bearing specimen (of the nature of holotype lectotype etc.) for Lampyris chinensis L. is extant, nomination of a specimen as a neotype is necessary to define this taxon objectively. Certain of the authors have examined the Linnaean collection in London (LB) and the Kiesenwetter collection in Munich (Jeng) and no types were found. It has not been possible to determine accurately the type locality of this species and Fu has collected without success in the south east area of China where Jeng believes the original population may have been taken (that being the area where persons who would pass on their material to Linnaeus were active). The specimen designated here belongs to a population easily accessible in Wuhan (the third largest city in China) and for which data exists on flashing patterns, morphological characters of males, females and larvae (see above), and which is clearly differentiated from other populations by the characters and diagnosis listed above. The brief original description indicated only that Lampyris chinensis L. was yellowish brown with dark elytral apices. This is consistent with several species described and distinguished here.
Remarks. As a working hypothesis we have accepted the table of synonymy given in Jeng et al. (2003:258) reproduced above, and our identifications of specimens as chinensis is consistent with their characterisation of populations on Taiwan (as Luciola praeusta ). However is not possible to confirm the identities of most of the other references to praeu sta in this table. The location of type specimens for praeusta has not been established (no types exist in the Kiesenwetter collection in Munich). A survey of the old literature indicates that praeusta was probably based on specimens of L. chinensis (L.) but in the absence of a type for chinensis it is not possible to either confirm or disprove that.
The Apostles of Linnaeus were a group of students who carried out botanical and zoological expeditions throughout the world that were either devised or approved by botanist Carl Linnaeus. The expeditions took place during the latter half of the 18th century with students designated 'apostles' by Linnaeus (http://en.wikipedia.org/ wiki/Apostles_of_Linnaeus accessed 12 November 2012). Jeng believes Linnaeus received most of his material from Olof Torén, Pehr Osbeck or Anders Spasrrman who frequently visited Guandong and other provinces in SE China. Fu and others attempted collections in this area but did not discover any specimens that could have been assigned to L. chinensis .
With the exception of Jeng et al. (2003) none of the following would have examined critical morphology like the aedeagal sheath which has only been in use in lucioline taxonomy since Ballantyne (1987a, b). Clearly it is hoped that our characterisation will give others the information to make wider collections and confirm or challenge our interpretations.
Check lists usually give no basis for their considerations and follow actions that preceded them e.g. Olivier (1902, 1907), Okada (1931), and Miwa (1931) and to some extent McDermott (1966). Others especially Lai et al. (1998) and Jeng et al. (1999) were reconsidered in detail in Jeng et al. (2003), as was Chen (2003) and form the basis for our identifications.
The synonymy with Luciola gorhami Ritsema appears to have been made without further type examination, but is based on what previous authors (Gorham, Olivier and McDermott) had determined. Gorham’s articles (1883, 1895, 1903) are unhelpful apart from indicating many species probably share this colour pattern, as did Olivier (1913), who described L. gorhami male abdomen as yellow except for the black 4th segment (= V5), consistent with many chinensis described here, and also Abs. perplexa .
Gorham (1880) highlighted the problems in attempting to identify similarly coloured species and distinguished six species with similar dorsal coloration including a reference to chinensis with black tibiae from Foochow, which he considered close to Luciola substriata (which has elytral punctures in lines). Of the rest praeusta Eschsch. was transferred to Colophotia and malaccae to Pteroptyx (McDermott 1966) . McDermott (1962:27) illustrated the genitalia of an Indian population which seems to be consistent with all the species we discuss here in having LL fused along their mid dorsal length, short produced areas at the posterolateral corners, and ML less than LL length.
Where morphology and life history from a well defined locality were described future corroboration of identity may be possible. It is possible, but as yet unproven, that most of the Indian specimens listed here could be found to belong to Abs. perplexa . Fletcher (1919) considered L. gorhami one of the common fireflies at Pusa, and described some features of the larva, which was found often under rotting leaves between May and August, and in captivity refused living food. The description of the tubercles along the posterior margins of most terga is consistent with our description. Mehta (1932) distinguished L. gorhami from Lahore from L. vespertina (= L. chinensis ) by leg colour, none of which is consistent with what we describe here. Saxena (1953a, b, 1957) described internal anatomy of L. gorhami but did not specify the population which might have been from Patna. Ganguly (1980) considered L. praeusta one of the most common fireflies in N. India (at Patna), with adults appearing in great numbers in the rainy season (July–September) in marshy areas, and hibernating as a larva. The extent of the male light organ, although described on page 2 as occupying the ventral surface of the last three abdominal segments, is depicted in Fig. 3 View FIGURE 3 page 3 in V6 and 7 only, with a wide clear lateral and posterior margin, inconsistent with the specimens we describe here. Ganguly’s Figs 1 View FIGURE 1 , 2 View FIGURE 2 are stylised dorsal views of adults appearing to indicate elytral punctation in lines. Barua et al. (2009) recorded emission spectra for a local population of L. praeusta (tentative identification by LB reassessed here and species assigned to Abs. perplexa ). Presently Indian government regulations do not permit borrowing of specimens, and most identifications can only be based on dried pinned specimens in external museums.
Of the ten abdominal colour patterns identified here at least two patterns are found in each area examined (where number of specimens exceeds 1), and three in Tiantang Zhai, Luotian, and Mt Tianmu. Fu did not collect Abs. chinensis and Abs. terminalis in the same area.
Jeng observed in Taiwan that both Abs. chinensis and Abs. anceyi prefer flying high, usually over, on or just below tree canopy in forests. Their males can produce flash trains in flight, about 9–11 quick flashes in 1–1.5 sec, followed by a long silence of several seconds. Females of chinensis also fly high in mating course, but they are more commonly seen flying low to lay eggs later in the season. It appears females of both chinensis and anceyi produce continuous glow after mating, since such bioluminescent display is more common in the late but not starting season. In general, it is quite easy to determine which is which by their habitats as well as male flash patterns in the field. It appears this is not the case in China.
TABLE 5. Measurement of male Abs. chinensis (L.) (in mm)
| 0.15–0.0 | 1.90–2.15 | 0.30–0.35 | 0.35–0.50 | 3.00–3.60 | 7.25–8.15 | 1.25–1.40 | 2.25–2.60 | 5.90–6.80 | 2.35–2.70 | Daguisi National Forest Park, Suizhou City |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.15–0.20 | 1.80–2.10 | 0.30–0.35 | 0.35–0.45 | 3.30–3.75 | 6.85–8.10 | 1.20–1.40 | 2.00–2.40 | 5.60–6.80 | 2.10–2.65 | Tiantangzhai, Mountain Dabie, |
| 0.15–0.20 | 1.85–2.00 | 0.30–0.35 | 0.40–0.45 | 3.15–3.35 | 5.70–6.30 | 1.15–1.25 | 2.10–2.35 | 5.70–6.30 | 2.25–2.50 | Wangjia Village, Hehua town, Yua nan County |
| 0.15–0.20 | 1.85–2.15 | 0.25–0.40 | 0.35–0.50 | 3.35–3.70 | 7.10–8.30 | 1.20–1.55 | 1.90–2.60 | 5.70–6.80 | 2.10–2.70 | Luotian Country, Huanggang City |
| 0.15–0.20 | 1.80–2.15 | 0.25–0.35 | 0.35–0.40 | 3.25–3.70 | 6.95–7.80 | 1.25–1.45 | 2.00–2.05 | 5.60–6.40 | 2.15–2.60 | Mountain Tiantai |
| 0.15–0.20 | 1.90–2.10 | 0.30–0.35 | 0.40–0.45 | 3.55–3.65 | 6.95–8.10 | 1.25–1.45 | 2.10–2.60 | 5.50–6.80 | 2.30–2.65 | Mountain Dongfang |
| 0.15–0.20 | 1.20–2.15 | 0.25–0.35 | 0.35–0.45 | 3.30–3.80 | 6.60–8.30 | 1.30–1.60 | 1.80–2.50 | 5.30–6.80 | 2.00–2.80 | Mountain Tianmu |
| 0.15 | 2.10 | 0.35 | 0.75 | 3.40–3.50 | 7.70 | 1.40 | 2.40 | 6.30 | 2.50 | Loudi City |
| 0.1.8–2.0 | 0.25–0.35 | 0.15–0.2 | 0.35–0.45 | 3.15–3.45 | 5.8–6.4 | 1.15–1.25 | 1.9–2.1 | 5.75–6.25 | 2.25–2.5 | Mt Sushansi |
TABLE 6. Measurement of female Abs. chinensis (in mm)
| ASD | ASW | GHW | SIW | Antennae length | Body length | Pronotal length | Pronotal width | Elytron length | Body width | Specimen Information |
|---|---|---|---|---|---|---|---|---|---|---|
| 0.35 | 0.20 | 1.50–1.55 | 0.40–0.50 | 3.30–3.80 | 7.70–8.35 | 1.40–1.60 | 2.35–2.50 | 6.30–6.90 | 2.45–2.75 | Tiantangzhai, Mountain Dabie |
| 0.30–0.35 | 0.15–0.20 | 1.40–1.95 | 0.35–0.55 | 3.30–3.80 | 7.25–8.50 | 1.20–1.60 | 2.00–3.00 | 6.00–7.10 | 2.00–3.00 | Luotian County, Huanggang City |
| 0.35 | 0.20 | 1.50 | 0.40 | 3.10 | 7.85 | 1.45 | 2.55 | 6.40 | 2.70 | Yidu County, Yichang City |
| 0.30 | 0.15–0.20 | 1.50–1.65 | 0.35–0.40 | 3.30 | 7.60–8.15 | 1.40–1.55 | 2.40–2.65 | 6.20–6.60 | 2.45–2.75 | Mountain Tiantai |
| 0.30–0.35 | 0.20 | 1.40–1.70 | 0.40–0.45 | 3.50–3.85 | 7.75–8.55 | 1.30–1.70 | 2.50–2.85 | 6.30–7.00 | 2.70–2.90 | Mountain Dongfang |
| 0.30–0.35 | 0.15–0.20 | 1.45–1.60 | 0.35–0.50 | 3.60–3.80 | 8.00–8.90 | 1.40–1.70 | 2.30–2.90 | 6.60–7.30 | 2.60–2.95 | Mountain Tianmu |
| 0.35 0.3 | 0.20 0.15–0.2 | 1.60–1.70 1.5–1.65 | 0.50–0.55 0.35–0.4 | 3.60–3.90 3.35–3.55 | 8.10–8.25 7.25–7.65 | 1.45–1.55 1.3–1.7 | 2.95–3.00 2.35–2.55 | 6.60–6.80 6.2–6.6 | 3.0–3.10 2.45–2.75 | Loudi City, Hunan Province Mt Sushansi |
TABLE 7. Development of larval Abs. chinensis under 25 ° C, photoperiod 12: 12.
| Instar | Number (n) | Body length (mm) * Body width (mm)* | Head width (mm)* | Instar period (d) * |
|---|---|---|---|---|
| 1st | 15 | 3.08±0.35 0.52±0.06 | 0.12±0.02 | 14.3±1.4 |
| 2nd | 16 | 4.29±0.25 0.90±0.05 | 0.19±0.01 | 13.2±2.0 |
| 3rd | 16 | 5.80±0.67 1.19±0.13 | 0.25±0.02 | 14.3±5.8 |
| 4th | 15 | 8.55±1.03 1.58±0.13 | 0.37±0.03 | 14.1±5.4 |
| 5th | 13 | 10.62±1.10 2.31±0.19 | 0.41±0.03 | 77.2±41.6 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Luciolinae |
|
Genus |