Liolaemus cuyumhue, Avila, Luciano Javier, Morando, Mariana, Perez, Daniel Roberto & Sites, Jack W., 2009
|
publication ID |
https://doi.org/10.5281/zenodo.190368 |
|
DOI |
https://doi.org/10.5281/zenodo.5632893 |
|
persistent identifier |
https://treatment.plazi.org/id/9E4AFC62-FFBE-1A20-B8B3-DCBECE23934B |
|
treatment provided by |
Plazi |
|
scientific name |
Liolaemus cuyumhue |
| status |
sp. nov. |
Liolaemus cuyumhue sp. nov.
Figure 1 View FIGURE 1 .
Type material. Holotype: MACN 38981 ( Fig. 1 View FIGURE 1 ), an adult male from sand dunes near Ruta Provincial 7, 28.7 km NW Añelo, Añelo Basin, Añelo Department, Neuquén Province, Argentina ( 38° 11’ S, 69° 01’ W, 259 m), collected 2 February 2003 by L. J. Avila, M. Morando, C. H. F. Perez, and K. Dittmar.
Paratypes ( Fig. 2 View FIGURE 2 ): FML 17592, LJAMM 4520–1 (males), from sand dunes, southern edge of Ruta Provincial 7, ( 38° 13' S, 68° 57' W, 260 m), Añelo Department, Neuquén Province; 15 November 2003; collected by D. R. Perez, J. Perez, M. Perez, and M. Perez Carrió; FML 17594, MACN 38982–3, MLP.S 2587, 2591 (males), FML 17593, LJAMM 3692, MACN 38984, MLP.S 2586, 2588/9–90 (females) from sand dunes near Ruta Provincial 7, ( 38° 13' S, 68° 57' W, 258 m), Añelo Department, Neuquén Province; 10 February 2006; collected by D. R. Perez, C. de la Vega and D. Zuñiga.
Diagnosis. Liolaemus cuyumhue is a member of the wiegmannii group of Liolaemus lizards, characterized by the presence of two or more rows of lorilabials scales rather than one between subocular and supralabilals, smaller in size than in other Liolaemus ; flat or concave rather than convex infralabials, mental scale narrower anteriorly than posteriorly; and with the exception of L. cuyanus and L. mapuche , six scales in contact with the mental ( Etheridge, 1995, 2000; Abdala, 2002). Liolaemus cuyumhue is distinguished from L. riojanus because its background coloration is yellow-cream to a light red-brick tonality rather than orange-brick or light ochre; dorsal spots are larger than in L. riojanus , and usually have few blue iridescent scales distributed on the dorsolateral areas of the body, rather than mostly grouped into small clumps of 2–4 scales on the sides of the body. L. cuyumhue never have suprascapular spots and series of dorsolateral yellow spots as is observed in some populations of L. riojanus . Another difference from L. riojanus , females of L. cuyumhue always lack precloacal pores. L. cuyumhue males are smaller than L. multimaculatus males (61.7 mm vs 72 mm SVL), with smaller dorsal scales, and fewer scales around midbody (62–71 vs 68–92). L. cuyumhue lack the dark brown/black scapular spots always present in L. multimaculatus . L. cuyumhue have smaller circular brown spots, speckles of smaller white dots, and a low density of blue iridescent scales distributed on the dorsolateral areas of the body instead of rhomboidal to circular gray, tan, or brown spots, speckles of white scales, and blue iridescent scales scattered irregularly on a gray or brown background, observed in L. multimaculatus . L. cuyumhue never have the general coloration of grey and blue scales observed in L. rabinoi , and never have iridescent blue scales clumped into small patches of 3–11 scales each on the sides of the body. Liolaemus cuyumhue lack the sexual dichromatism observed in L. wiegmannii , have smooth or slightly keeled dorsal head scales instead of rugose and protruded, dorsal narines instead of lateral, more supralabial (8–11 vs 4–6) and infralabial scales (7–10 vs 5–7), and smaller body scales (midbody scale counts 64–71 vs 38–60, dorsal body scale counts 78–90 vs 42–62). L. wiegmannii females sometimes have precloacal pores (0–6) but these are completely absent in L. cuyumhue females. Dorsal scales in L. cuyumhue are smooth to slightly keeled and juxtaposed to slightly imbricated instead of strongly keeled and imbricated as in L. wiegmannii . L. cuyumhue males lack dorsolateral and lateral dark spots, paravertebral lines, numerous and larger blue scales in lateral areas, and bright yellow or orange scales, all characteristics of L. wiegmannii . L. cuyumhue always lack pre and postscapular spots or paravertebral lines observed in L. azarai , body size is larger (maximum SVL in males 61.7 vs 54.3 mm; in females 59.9 vs 48.7 mm), and have smaller body scales (midbody scale counts 64–71 vs 32–42, dorsal body scales 78–90 vs 42–60). L. cuyumhue males have higher number of precloacal pores (7–9 vs 5–6) but in females these always are absent ( 2–3 in L. azarai females). L. cuyumhue is slightly larger than L. arambarensis (maximum SVL 60 vs 56 mm in males, 54.3 vs 48.7 mm in females), have smaller dorsal scales, with more scales around midbody (64–71 vs 60–66), and along the trunk (78–90 vs 57– 64). L. cuyumhue lack a mid-dorsal white line and two dorsolateral stripes, with two series of paravertebral brown marks resembling triangles bordered by a white bar, as observed in L. arambarensis . L. cuyumhue is considerably smaller than L. lutzae (males 61.7 mm vs 84.0 mm, females 59.9 mm vs 69.0 mm), dorsal coloration lacks the conspicuous wide vertebral band bordered with dark paravertebral spots, and wide gray dorsolateral stripes observed in L. lutzae . L. cuyumhue is smaller (males 61.7 mm vs 70.0 mm, females 59.9 mm vs 60.0 mm), lacks strongly keeled and imbricate dorsal scales, lacks a dark longitudinal bar above the forelimb insertion, and lacks the ventral sexual dimorphism observed in L. occipitalis . L. cuyumhue is smaller than L. salinicola and L. scapularis (61.7 vs 76 vs 77 mm in males, 59.9 vs 68 vs 65 mm in females) and lacks stripes and gray throat observed in L. salinicola and the scapular mark characteristic of L. scapularis .
Description of the holotype. Adult male. SVL 51.2 mm, total length 111.2 mm. Axilla groin distance 23.4 mm. Head width 10.8 mm, head length 12.9 mm, head height 6.6 mm, snout length 4.8 mm, horizontal diameter of orbit 2.5 mm, internarinal distance 3.3 mm; eye-nostril distance 1.9. Arm length 15.1 mm, tibial length 11.5 mm, foot length 15 mm (all from the right side). Dorsal head scales smooth, flat to slightly concave, a few pitted with scale organs. Eighteen dorsal head scales (from a line drawn horizontally between anterior margin of external auditory meatus to anterior margin of rostral). Rostral pentagonal, wider that high (1.9 x 0.8 mm). Two postrostrals, wider than high; together with anterior lorilabials separate nasal scales from rostral. Nasal scales longer than wide (1.4 x 0.9 mm), dorsolateral in position. Nostril almost rounded in shape, occupying slightly less than half of nasal scale. Nasal scales in contact with nine scales (left) and eight scales (right). Internasal scales in two rows; two anterior, small, and pentagonal preinternasals; three posterior post-internasals, longer than wide, medial slightly larger than laterals. Two small postnasals behind each nasal. Ten frontonasals, irregular in shape, in two irregular rows. Nine prefrontals in two rows, five scales in front, four scales in back. Two scales conspicuously larger in each row, lateral in position in the first row, medial in the second row. Three frontal scales, median longer than wide, almost equal in size to prefrontals. Nine frontoparietals, irregular. Interparietal with a conspicuous parietal eye, oblong, surrounded by eight smaller irregular scales. Parietals smooth and irregular, variable in size. Eighteen circumorbitals on left side, twenty two on right side. Supraoculars small, irregular, numerous, 59 left, 46 right, five conspicuously larger, wider than high on each side. First canthal higher than wide, posterior canthal longer than wide, non overlapping first superciliary. A well marked but blunt canthal ridge. Six superciliaries on each side, overlapped. Loreals 9–8; flat to slightly convex. Loreals, preocular, and lorilabials forming a conspicuous concavity. Lorilabials in two-three rows, slightly bulged, becoming smaller below subocular, none wider than supralabials. Upper ciliary scales in two rows, those of inner row flat and quadrangular; those of outer row rectangular and conspicuously projecting. Lower ciliaries more similar in size and shape than upper ciliaries, some conspicuously projecting. Palpebral scale small, irregular, flat. Preocular small, quadrangular, with a keel. Subocular elongated (4.4 x 0.4 mm), with a distinct and sharp keel. Supralabials 8-8, last three on right side, distinct, two times longer than high. Temporal scales roughly quadrangular, higher than large. Supratemporals smaller than temporals, roughly rounded, a few almost granular. Nuchals slightly keeled, juxtaposed, rounded. Lateral nuchals small, almost granular, non-imbricated. Occipital scales small, irregular, flat, becoming granular distally. Auditory meatus oval, higher than wide (1.9 x 1.1 mm), surrounded above, behind, and below by granular scales. Pretympanic scales, rounded to obovate, non-projecting. Mental heptagonal (1.6 x 1.2 mm), wider than high, in contact with six scales: infralabials, postmentals, and sublabials. Infralabials flat 7–8, meeting sublabials at an acute angle. Chinshields slightly evident, two postmentals evident but following scales becaming similar to sublabials and gulars in the third row. First sublabials and sublabials in contact with infralabials, smaller than surrounding scales. Gulars imbricate, smooth, becoming rounded and notched distally. Longitudinal oblique and antegular folds distinct, gular and antehumeral well marked. Dorsal body scales obovate, subimbricated, with a blunt keel. Dorsal limb scales 2– 3 times larger than dorsal body scales, rhomboidal to obovate, imbricate, and bluntly keeled. Suprabrachials nearly two times larger than suprantebrachials, with a more regular rhomboidal shape and keel. Supraantebrachials becoming obovate near the insertion, a few with a small spine. Supracarpals and supradigitals smooth. Infrabrachials small, almost granular. Infraantebrachials 2–4 times larger than infrabrachials, nongranular. Infrabrachials oval to rounded, Juxtaposed near the infrabrachials, becoming progressively imbricated and obovate, some with a blunt keel, near the hand. Infracarpals blunt to well marked keel, imbricate, none tridentate. Subdigital lamellae of manus with three keels, numbering I:9, II:13, III:20; IV:20; V:12. Claws long and slender, slightly curved. Supracarpals smooth or with a blunt keel, supradigitals smooth. Infrafemorals and infratibials smooth, imbricated. Infratarsal keeled, strongly imbricated, none tridentate. Infradigitals unidentate, numbering: I:10; II:14, II:18, IV:24, V:14. Long and slender claws, slightly curved. Ventral scales lanceolate to rhomboidal, smooth, imbricated. Some scales with an apical notch. Ventral scales slightly larger than dorsal scales. Scales in the flanks between dorsal and ventral distinctly smaller. Scales in front, above and behind limb articulations small, granular to flat. Caudals imbricate, with a blunt but conspicuous keel, same size to the dorsal and ventrals, becoming smaller to the tip of the tail. Ventral caudals with a blunt keel. Scales of the cloacal apron slightly smaller that the ventral scales. Seven insconspicuous precloacal pores.
Coloration. Dorsal pattern on head, trunk, tail and limbs with brown rounded spots (4 to 9 scales in size) and speckles of cream, gray, tan, and reddish-brown on reddish background. Between 9 to 10 irregular transversal series of 5 to 7 spots between occiput and rump. Background coloration varies according to ambient light between a yellow-cream (full sun) to a red-brick tonality (in shade). Reddish-brown spots larger than other colored spots, sometimes white bordered anteriorly, posteriorly or both. Some larger creamy spots on dorsolateral areas of trunk. Head with brown spots smaller than in other areas of body; tail brown spots faintly larger than in limbs, head and trunk. Some clear lines faintly indicated on ciliar, supraocular, and longitudinal neck folds. Lateral areas of the body with larger brown spots on white background. Ventral areas immaculate cream-colored except in throat and lateral areas of chest and belly marked with brightly light brown to reddish-brown spots irregularly distributed. Some small, scattered blue iridescent scales are distributed on the dorsolateral areas of the body. In preservative same general coloration but faded, bright ventral spots become light gray, blue iridescent scales disappear.
Variation. Morphometric and scale variation is presented in Table 1 View TABLE 1 and 2 View TABLE 2 (with all other diagnostic features mentioned above), and dorsal and ventral color variation is depicted in Figure 2 View FIGURE 2 . As in other members of the wiegmannii group (except L. wiegmannii ), intrapopulation variation in morphology is not conspicuous. Postfemoral patch not present in females and variable in size in males, between 18–25 scales. Dorsal background color usually similar to holotype, recently captured adult males have a brighter coloration. Variation most frequently seen is in the distribution and size of dorsal and ventral spots.
Maximum SAM DS PP Scapular Dorsal sexual Iridescen Source
SVL marks dichromatism t blue
scales
F M F M
Sexual dimorphism. Females lack of the colored ventral spots characteristic of the males and the iridescent blue scales. The base of tail of males is expanded laterally, and cloacal opening is not rounded as is seen in females. Female lacks of the larger and obvious precloacal pores.
Etymology. Named in reference to the characteristics of the substrate of the type locality; cuyumhue is a Mapuche word meaning sandy place.
Geographic distribution. Liolaemus cuyumhue is known only from the type locality were the holotype and paratypes were collected, in the Añelo basin close to the Provincial Road 7, in Neuquén Province ( Figs. 3 View FIGURE 3 , 4 View FIGURE 4 ). Sand dune formations with suitable habitats for L. cuyumhue are restricted to the southern edges of the Añelo basin, but additional field work is necessary to explore other apparently suitable habitats north of this area. Liolaemus cuyumhue lives in marked isolation from other members of the L. wiegmannii group, the closest locality for L. multimaculatus is ~ 580 km SE (Vega and Bellagamba 1994); L. wiegmannii is found more than 300 km NE ( Tiranti and Avila 1997), L. rabinoi was described for sandy areas around the Nihuil Dam in Mendoza province, more than 400 km N ( Cei 1974, Etheridge 2000), and L. riojanus inhabit in dunes at least 700 km N ( Cei 1979, Etheridge 2000). Field surveys carried out from 2000 to 2008 in dunes systems around the Añelo basin revealed other Liolaemus species different from any related to the wiegmanni group.
Natural history. Liolaemus cuyumhue is known from an isolated sand dune system in the region known as Bajo de Añelo, in eastern Neuquén. This is a depression between the Auca Mahuida Plateau and the Colorado and Neuquén rivers, with its lowest point reaching 223 m. The geological origin of the Añelo depression is attributed to karst activity, but sand dunes are probably remnants of old sea shore dunes ( Uliana and Dellape 1981). The dunes are sparsely covered by clumps of Sporobolus rigens , Neosparton darwinii , Larrea divaricata , Prosopis flexuosa var depressa , and Atriplex zampa . Liolaemus cuyumhue is syntopic with other species of Liolaemus , including L. grosseorum , L. mapuche , L. gracilis , and in surrounding outcrops L. austromendocinus ; other lizard species such as Homonota fasciata , H. darwinii , Cnemidophorus longicaudus , Leiosaurus belli , and Pristidactylus cf fasciatus are also found in sympatry. The holotype was collected at mid afternoon on a cloudy day and was the only specimen of this species observe that day, while individuals of other species were normally active. Paratypes were collected late in the afternoon after the substrate temperature was cooler. We did not observed bimodal activity but probably this type of activity is common in summer on very hot days. L. cuyumhue is insectivorous and presumably oviparous, as are other members of the wiegmannii group.
Individuals of L. cuyumhue were observed only on bare or sparsely vegetated dunes with extensive areas of open sand. They appear not to extend out into the more vegetated sandy flats or rocky areas that usually border the dunes. Field observations of L. cuyumhue were made in February and November 2003, February 2006, and January 2007. When first observed, most individuals were close to clumps of vegetation with a few observed basking on open areas between the clumps. Lizard observation was very difficult because their coloration usually matches soil color, and usually we detected them only when they were running from our approach. Their first escape behavior was to flee into the clumps and remain motionless on the surface. If pursuit continued, they would move to another location and again remain immobile on the surface. Only if pursued multiple times would lizards bury into the sand. We did not ever observe such a burial, but we presume that this behavior is likely very similar to the description made for L. multimaculatus (a closely related species) by Halloy et al. (1998). They usually “disappear” in a clump of vegetation with no evidence of a burrow entrance.
Liolaemus cuyumhue lives in a region were the oil and gas companies conduct some of the more intensive operations in Argentina; new rigs, tracks and roads are opened regularly; frequently modifying some areas with suitable habitats for L. cuyumhue that apparently do not cover large areas in the Bajo de Añelo. How the oil activity will affect this species must await further study. The entire wiegmannii group is threatened by human activities, urban and tourist development are rapidly fragmenting coastal sand dunes in Buenos Aires province, the habitat of L. multimaculatus ( Vega et al. 2000) ; tourist activities also destroy the habitat of L. lutzae ( Rocha and Bergallo 1992) and L. occipitalis ( Di Bernardo et al. 2000) ; industrial agriculture (vineyards, olive groves), road construction and deforestation have destroyed extensive areas of habitat for L.
salinicola and L. scapularis (Avila, unpublished data); a large dam on the Rio Paraná probably destroyed large areas inhabited by L. azarai (Avila 2003) ; and L. rabinoi is considered by some authors as extinct, probably by habitat degradation as a result of human alteration after construction of El Nihuil Dam (Cei 1986, Etheridge 2000). We hope that this destiny will not be the future for our new species.
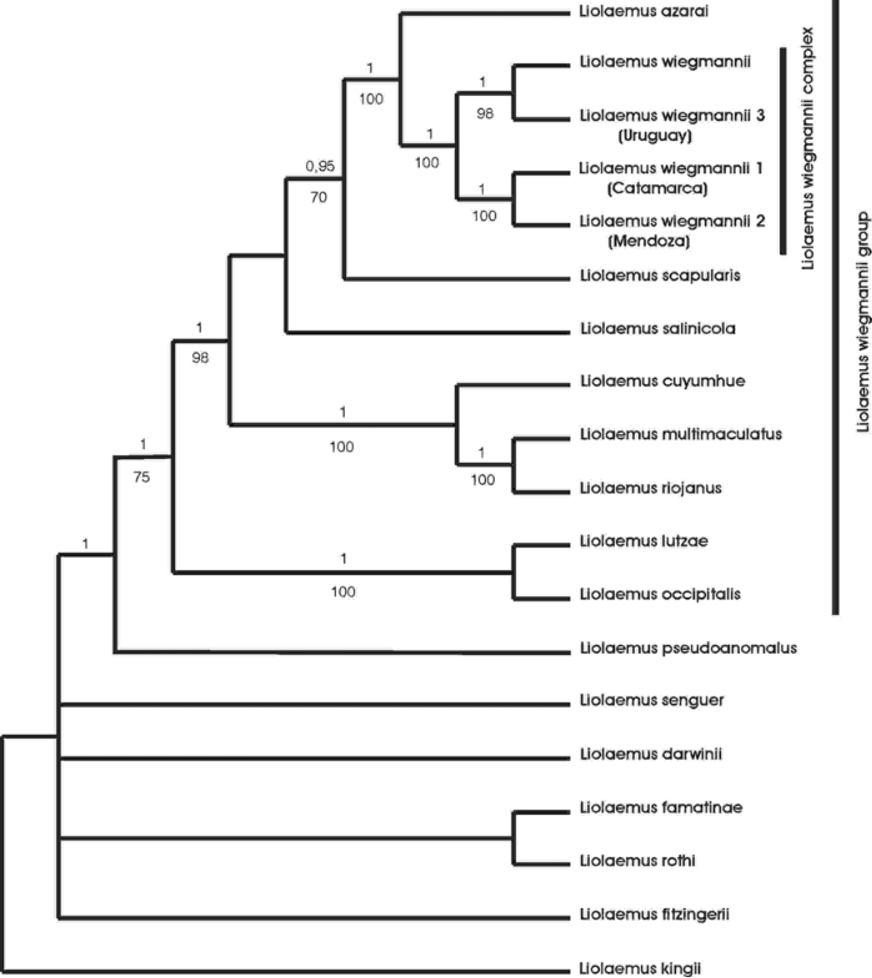
Phylogenetic affinities. Liolaemus cuyumhue is the only species of the wiegmannii group recorded from Neuquén province. The analyses of the separate gene partitions (Cyt-b, ND4, 12S and C-mos) showed some incongruences, but the majority were between the outgroups, and in most cases these relationships were not resolved and the outgroups formed a polytomy basal to the wiegmannii group. Most of the relationships among the ingroup taxa were consistent with the different partitions. The clades ( L. wiegmannii complex + L. azarai ) and ( L. cuyumhue ( L. multimaculatus + L. riojanus )) were recovered with the four genes. These two clades plus L. scapularis and L. salinicola formed a monophyletic group with the three mitochondrial genes. Liolaemus lutzae and L. occipitalis were recovered as sister taxa with the mitocondrial markers, but we did not have C-mos sequence for L. lutzae to evaluate this concordance. Liolaemus pseudoanomalus was recovered as the sister taxon of the L. wiegmannii group with all mitochondrial markers. Considering this high level of congruence within the ingroup we only present the phylogenetic tree obtained with the combined data set ( Fig. 5 View FIGURE 5 ). We found strong evidence for the monophyly of the wiegmannii group (posterior probability [pp] = 1.0 and MP bootstrap [MPb] = 75%). This result is congruent with the molecular hypothesis proposed by Schulte et al. (2000) and Cruz et al. (2005), and with the morphological hypothesis of Etheridge (2000). We also found some support for L. pseudoanomalus as the basal taxon of the wiegmannii group (pp=1.0), this relationship was also recovered by Cruz et al. (2005) and Schulte et al. (2000). The new species described here, Liolaemus cuyumhue , is nested within the wiegmannii group and is the sister taxon of the ( L. multimaculatus + L. riojanus ) clade with high support (pp=1.0; MPb=100%). Another monophyletic group recovered with high support is the wiegmannii complex + L. azarai (pp = 1.0, MPb = 100%). Also with high support (pp = 1.0, MPb = 100%) Liolaemus lutzae was recovered as the sister taxon of L. occipitalis ; the same relationship was found by Schulte et al. (2000), but Etheridge (2000) found these two species more distantly related. L. rabinoi is probably related to L. cuyumhue ; it has not been found after its original description in sand dunes around El Nihuil in Mendoza province, a locality 300 km N of Añelo sand dunes, and is probably extinct now ( Etheridge, 2000). Our field survey of the type locality did not produce any lizards so tissues for molecular analysis are not available, rendering its phylogenetic relationships with other members of the wiegmannii group uncertain. L. arambarensis was previously confused with L. wiegmannii and Verrastro et al. (2003) suggested a close relationship with a clade formed by ( L. rabinoi + (L. multimaculatus + L. riojanus )) based on morphological information provided by Etheridge (2000). Table 3 View TABLE 3 lists the uncorrected cytochrome b pairwise differences between all the taxa included in this study. Further research is needed to propose a well-supported hypothesis of the relationship of L. arambarensis within the wiegmannii group.
TABLE 1. Variation in squamation and morphometric characteristics of paratypes of Liolaemus cuyumhue. Measurements are in mm, numbers for each variable indicate mean ± standard deviation, with range in parentheses. Precloacal pores, infra and supralabials scales are shown as ranges..
| Variable | Males (n = 9) | Females (n = 7) |
|---|---|---|
| SVL Head length | 52.8 ± 6.4(42.7–61.7) 12.5 ± 1.3(10.8–15.0) | 55.3 ± 3.3(50.82–59.9) 12.6 ± 0.5(12.1–13.3) |
| Head width | 10.8 ± 0.9(9.4–12.4) | 10.7 ± 0.53(10.0–11.6) |
| Arm length Tibial length | 15.9 ± 1.8(13.4–17.9) 11.1 ± 1.10(9.3–12.3) | 16.4 ± 0.44(15.5–16.9) 10.9 ± 0.48(10.0–11.5) |
| Foot length | 19.1 ± 1.3(18.0–22.0) | 16.0 ± 0.63(15.0–16.8) |
| Axilla-groin distance Midbody scales | 21.6 ± 3.2(16.3–27.2) 67.2 ± 2.4(64–71) | 24.7 ± 2.3(21.5–27.9) 67.1 ± 1.0(66–69) |
| Dorsal scales | 84.7 ± 3.7(78–90) | 83.8 ± 2.4(82–88) |
| Ventral scales Pre-cloacal pores | 78.5 ± 4.5(74–89) 7–9 | 81.4 ± 3.4(77–87) 0 |
| Infradigital lamellae (3rd to hand) Infradigital lamellae (4th to foot) | 18.4 ± 0.7 (17–19) 23.2 ± 1.5 (22–26) | 17.6 ± 0.8 (17–19) 22.7 ± 1.1(21–24) |
| Supralabial scales Infralabial scales | 8–10 7–10 | 9–11 8–10 |
TABLE 2. Comparison of characters between the species of the L. wiegmannii clade. SAM = scales around midbody, DS = dorsal scales, PP = precloacal pores.
| L. arambarensis | 56 | 60 | 60–66 | 57–64 | 3–4 | 4–7 | no | evident | yes? | Verrastro et al. 2003 |
|---|---|---|---|---|---|---|---|---|---|---|
| L. azarai | 48.7 | 48.3 | 32–42 | 35–37 | 0–3 | 5–6 | yes | evident | yes | Avila 2003, this study |
| L. cuyumhue | 59.9 | 61.7 | 64–71 | 78–90 | 0 | 7–9 | no | absent | yes | This study |
| L. lutzae | 69 | 84 | 56–74 | 54–75 | 0 | 6 | no | absent | no | Etheridge, 2000 |
| L. multimaculatus | 64 | 72 | 68–92 | 79–118 | 0 | 5–11 | yes | absent | yes | Etheridge, 2000 |
| L. occipitalis | 60 | 70 | 67–79 | 75–86 | 0 | 7–10 | no | absent | no | Verrastro et al. 2003 |
| L. rabinoi | 68 | 61 | 73–81 | 92–105 | 0 | 5–9 | yes | absent | yes | Cei, 1974; Etheridge, 2000 |
| L. riojanus | 57 | 63 | 61–79 | 72–93 | 0–6 | 6–11 | yes | absent | yes | Etheridge, 2000; Laspiur et al. 2006 |
| L. salinicola | 68 | 76 | 58–77 | 69–97 | 0 | 6–10 | yes | absent | yes | Etheridge, 2000 |
| L. scapularis | 65 | 77 | 49–68 | 58–78 | 0–6 | 5–10 | yes | absent | yes | Etheridge, 2000 |
| L. wiegmannii | 60 | 59.1 | 38–58 | 43–67 | 0–6 | 4–8 | yes | strong | yes | This study |
TABLE 3. Cytochrome-b uncorrected pairwise genetic distances; bold letters identify comparisons between L. cuyumhue and the two least genetically distant species.
| Taxon 1 | Taxon 2 | Distance in % |
|---|---|---|
| L. riojanus | L. multimaculatus | 1.23 |
| L. wiegmannii 2 | L. wiegmannii 1 | 1.51 |
| L. cuyumhue | L. multimaculatus | 2.47 |
| L. riojanus | L. cuyumhue | 2.72 |
| L. wiegmannii | L. wiegmannii 3 | 2.84 |
| L. wiegmannii 2 | L. wiegmannii | 4.03 |
| L. wiegmannii | L. wiegmannii 1 | 4.20 |
| L. wiegmannii 2 | L. wiegmannii 3 | 4.52 |
| L. wiegmannii 1 | L. wiegmannii 3 | 4.81 |
| L. wiegmannii | L. azarai | 9.26 |
| L. occipitalis | L. lutzae | 9.26 |
| L. scapularis | L. multimaculatus | 9.51 |
| L. riojanus | L. wiegmanni 3 | 9.51 |
| L. wiegmannii 3 | L. multimaculatus | 9.75 |
| L. scapularis | L. cuyumhue | 9.75 |
| L. riojanus | L. scapularis | 9.75 |
| L. azarai | L. wiegmannii 1 | 9.88 |
| L. wiegmannii | L. multimaculatus | 9.88 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SuperFamily |
Iguania |
|
Family |
|
|
Genus |
Liolaemus cuyumhue
| Avila, Luciano Javier, Morando, Mariana, Perez, Daniel Roberto & Sites, Jack W. 2009 |
L. azarai
| Avila 2003 |
L. multimaculatus ( Vega et al . 2000 )
| Vega et al. 2000 |
L. occipitalis ( Di Bernardo et al . 2000 )
| Di Bernardo et al. 2000 |