Nanhaipotamon incendium, Huang & Mao & Shih, 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.5026.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:F69E5D4A-925D-46B3-BBA7-4712FF2E206B |
|
DOI |
https://doi.org/10.5281/zenodo.5275080 |
|
persistent identifier |
https://treatment.plazi.org/id/60AD742A-6170-4790-8563-4CC10722CFA4 |
|
taxon LSID |
lsid:zoobank.org:act:60AD742A-6170-4790-8563-4CC10722CFA4 |
|
treatment provided by |
Plazi |
|
scientific name |
Nanhaipotamon incendium |
| status |
sp. nov. |
Nanhaipotamon incendium View in CoL n. sp.
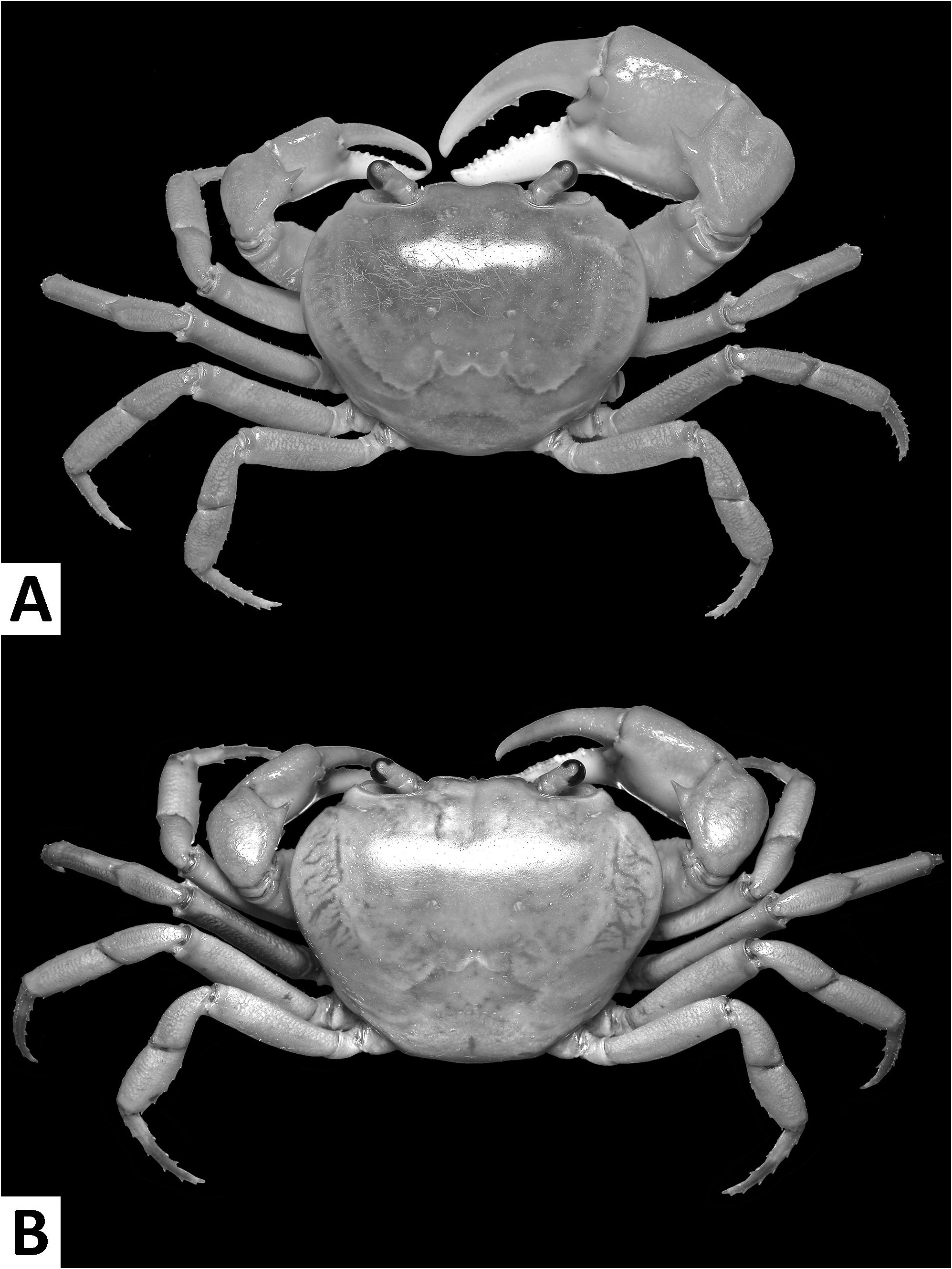
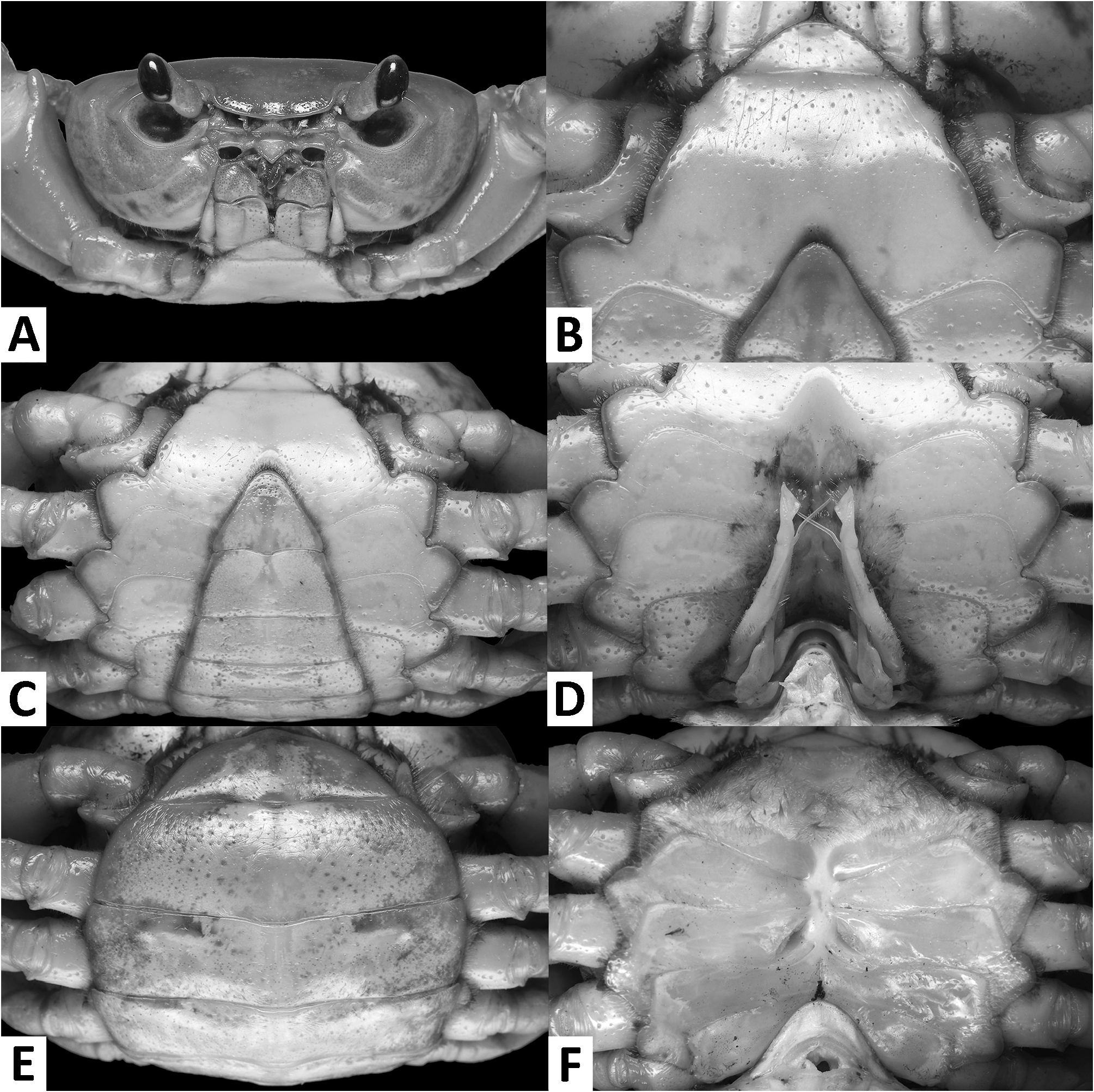
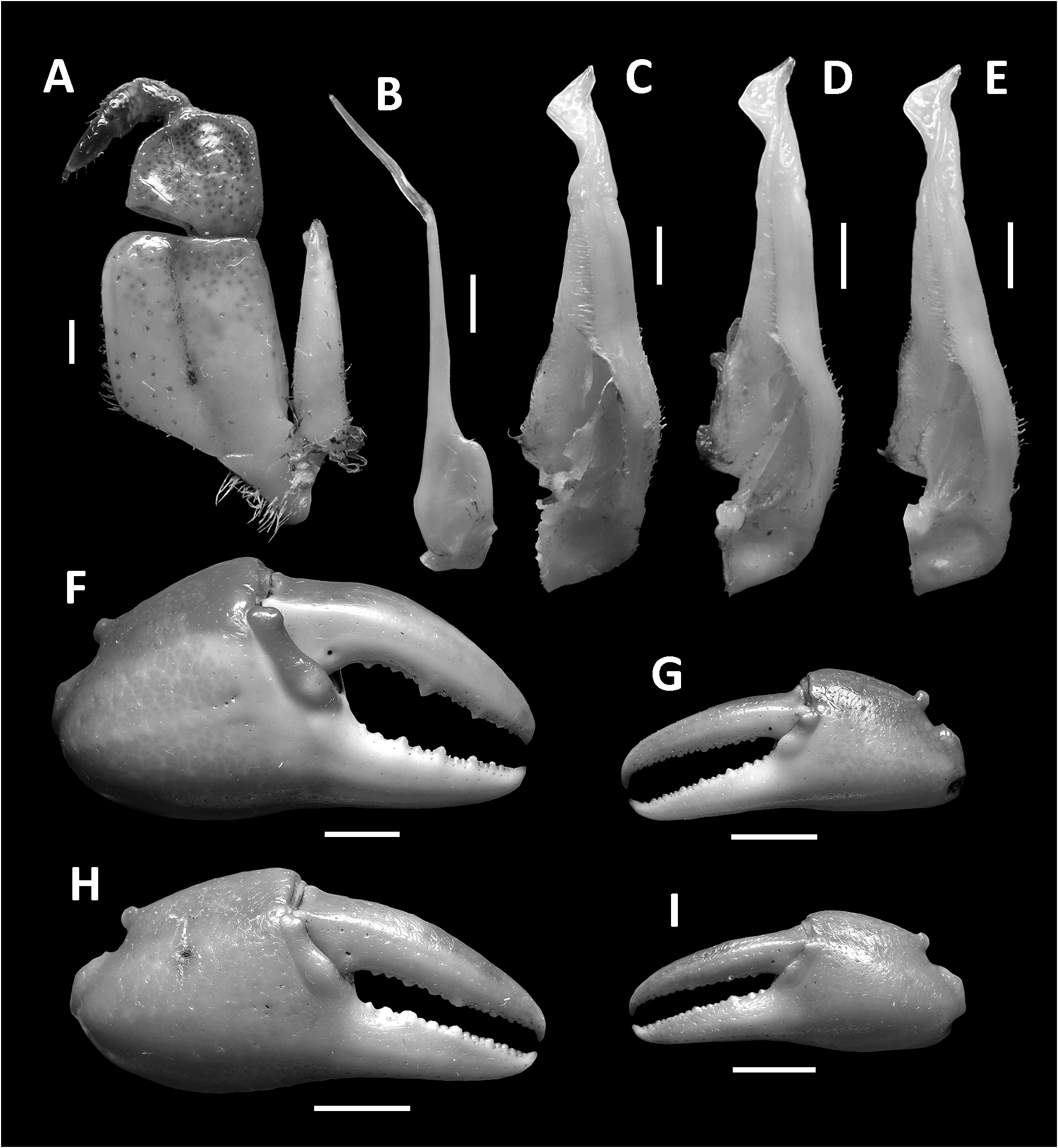
( Figs. 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 , 7I, J View FIGURE 7 , 8A View FIGURE 8 )
urn:lsid:zoobank.org:act:
Type material. Holotype: SYSBM 001799 , male (31.3 × 25.0 mm), Boluo County [ca. 23.3°N, 114.3°E], Huizhou City , Guangdong Province, China, shallow mud burrow, 700 m a.s.l., coll. C. Huang, August 2018. GoogleMaps
Paratypes: SYSBM 001801 , 001803 , 001804 , 3 males (27.0 × 21.6 mm, 25.4 × 20.5 mm, 18.7 × 15.3 mm), same data as for holotype GoogleMaps ; SYSBM 001800 , 001802 , 2 females (32.0 × 25.0 mm, 29.4 × 23.6 mm), same data as for holotype GoogleMaps ; SYSBM 001805 , male (25.2 × 20.2 mm), Boluo County, Huizhou City , Guangdong Province, China, shallow mud burrow, 700 m a.s.l., coll. W.H. Wang, August 2017 ; SYSBM 001806 , female (27.9 × 21.6 mm), same data as above; NCHUZOOL 17034, male (31.3 × 25.0 mm), Boluo County, Huizhou City , Guangdong Province, China, deep mud burrow with water at bottom, 700 m a.s.l., coll. C. Huang, November 2018 ; AM P.105614, male (29.1 × 23.8 mm), Boluo County, Huizhou City , Guangdong Province, China, shallow mud burrow, 700 m a.s.l. coll., C. Huang, August 2018 ; AM P.105614, female (32.7 × 26.6 mm), same data as above ; ZRC 2021.0415 View Materials , male (25.8 × 20.5 mm), same data as above ; ZRC 2021.0416 View Materials , female (25.6 × 21.0 mm), same data as above .
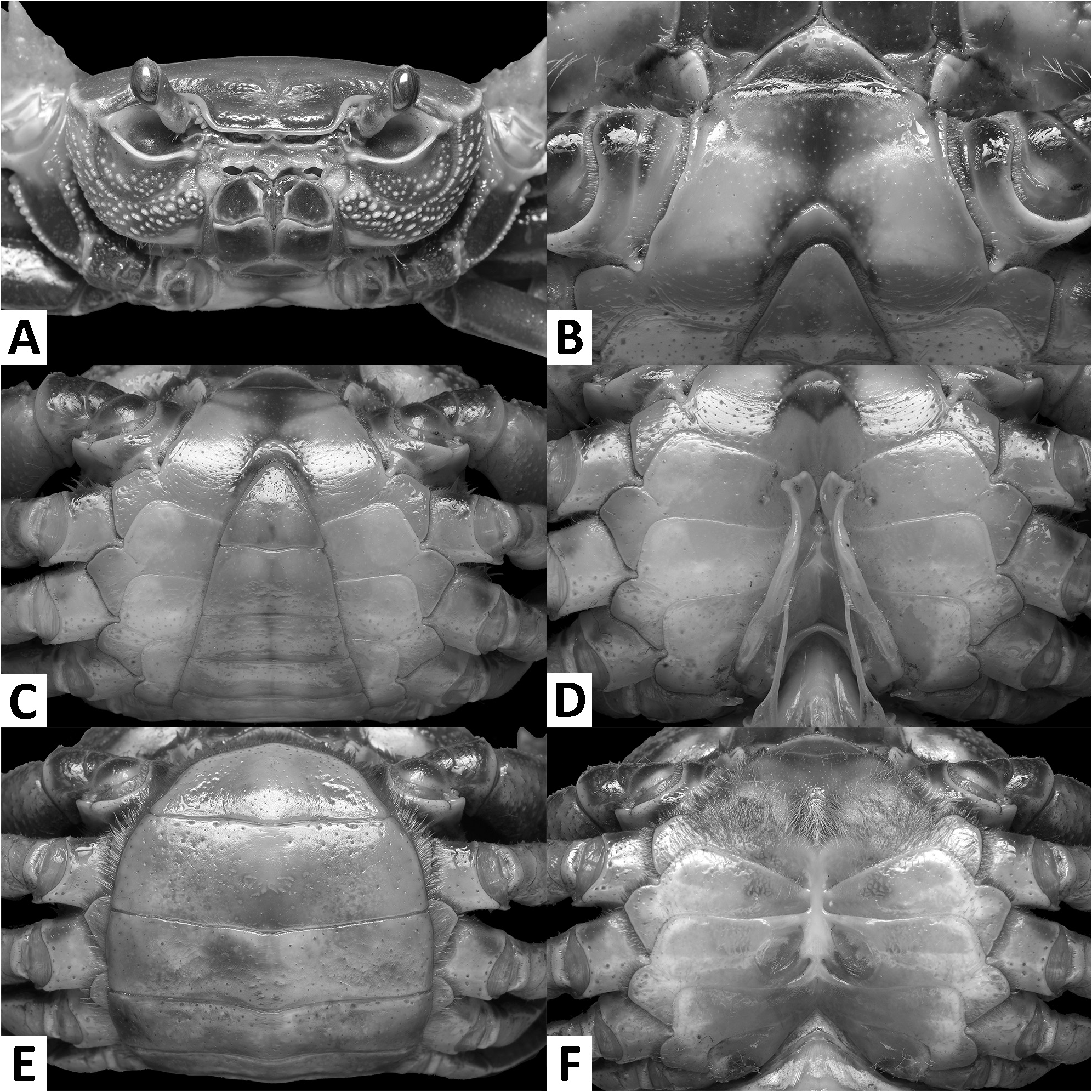
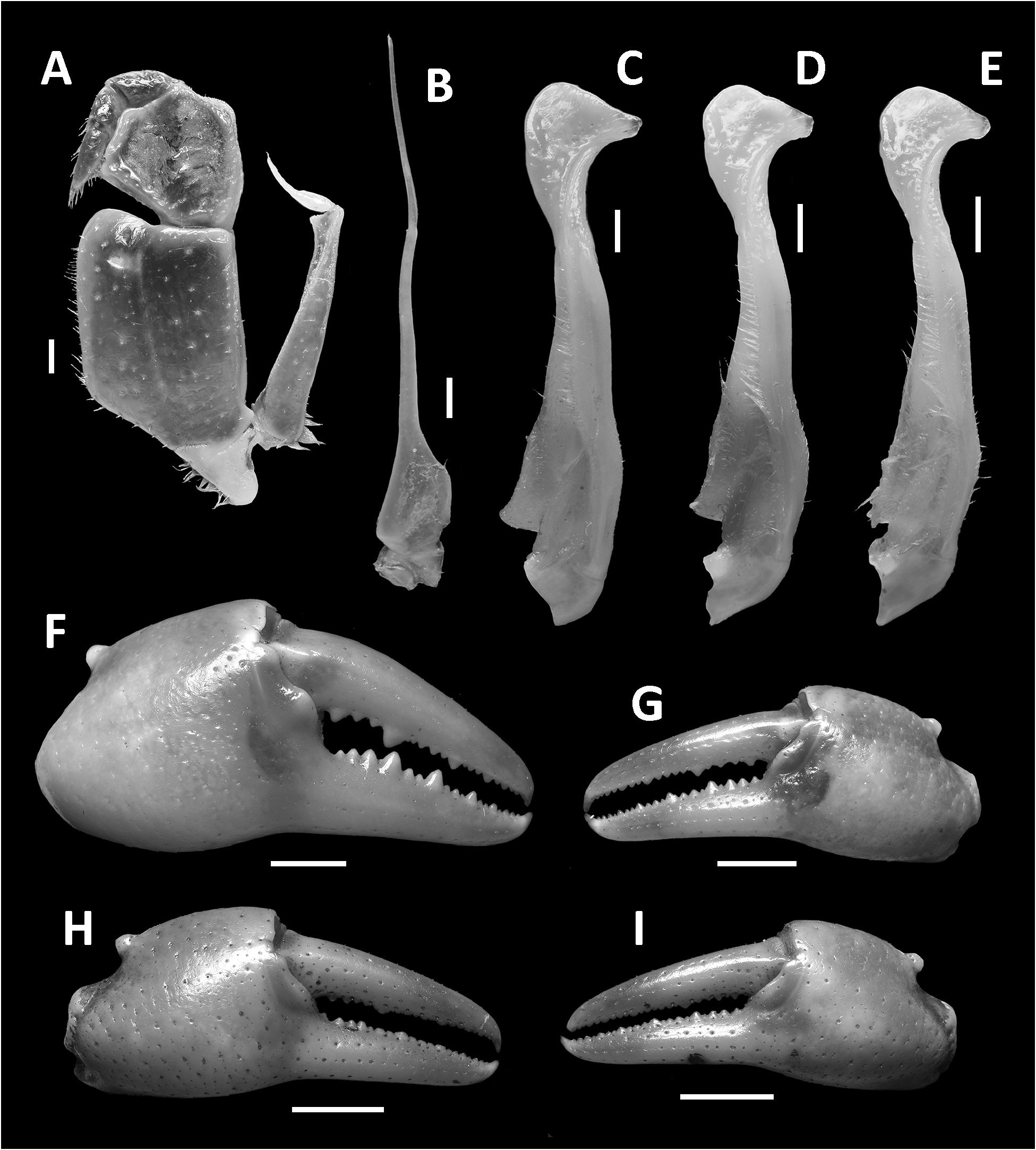
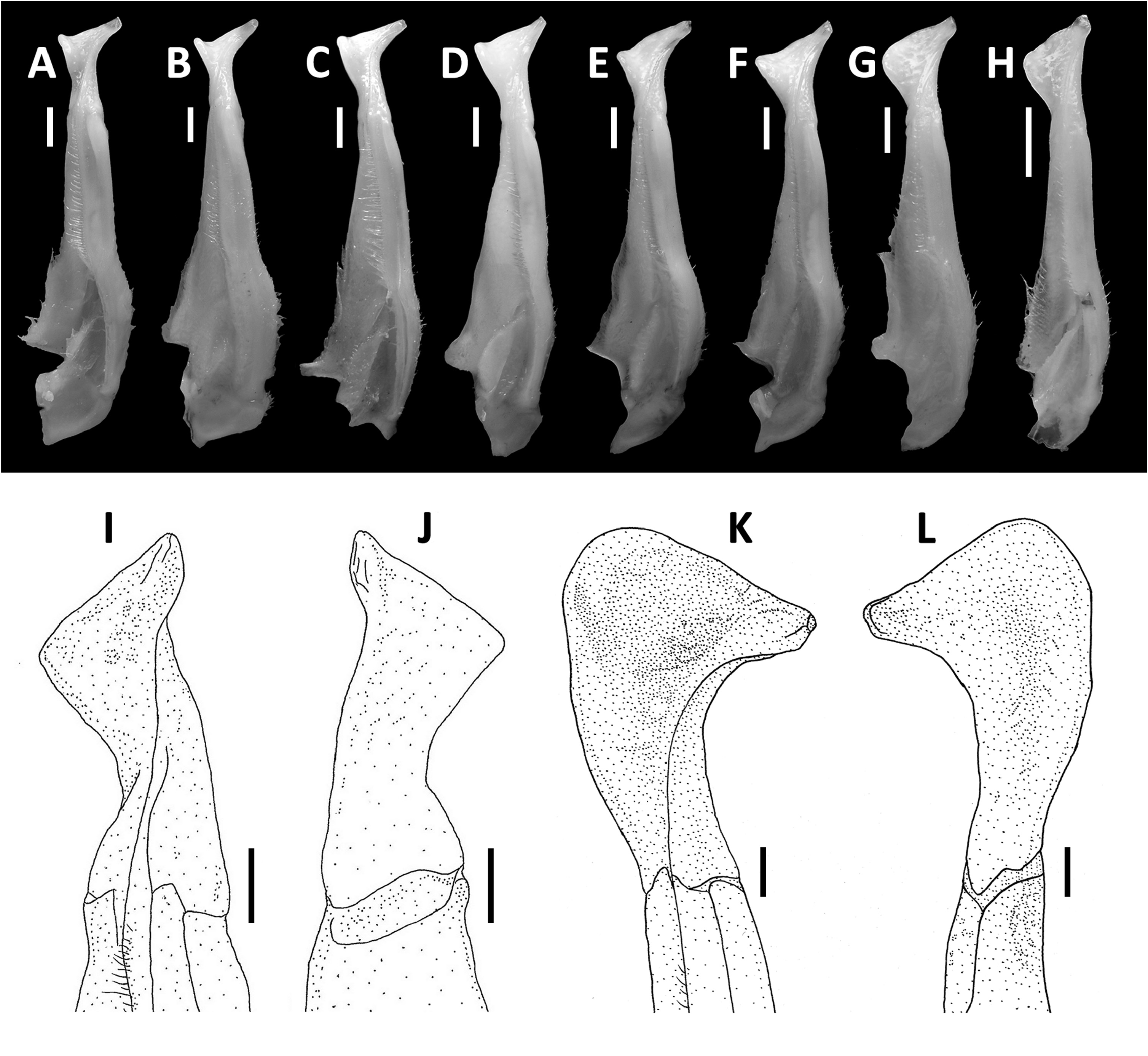
Diagnosis. Carapace broader than long, regions indistinct ( Fig. 1 View FIGURE 1 ). Dorsal surface smooth, finely pitted, convex ( Fig. 1 View FIGURE 1 ). Anterolateral margins smooth, lined with numerous indistinct granules ( Fig. 1 View FIGURE 1 ). Posterolateral surfaces smooth ( Fig. 1 View FIGURE 1 ). Sub-orbital, sub-hepatic and pterygostomial regions clearly divided by sutures, with smooth and pitted surface ( Fig. 2A View FIGURE 2 ). Maxilliped III exopod reaching to proximal one-fifth of merus, flagellum absent ( Fig. 3A View FIGURE 3 ). G1 slender, reaching beyond pleonal locking tubercle almost up to suture between sternites IV/V in situ ( Fig. 2D View FIGURE 2 ). G1 subterminal segment 2.6–2.7 × as long as terminal segment, tapering distally. G1 terminal segment relatively small, inverted foot-shaped; inner proximal margin strongly concave; inner distal margin almost straight to gently convex; apex acute, directed outward, orientation oblique to longitudinal axis of G1 ( Figs. 3C–E View FIGURE 3 , 7I, J View FIGURE 7 ). G2 subterminal segment 2.0–2.1 × as long as flagellum-like terminal segment (n = 3) ( Fig. 3B View FIGURE 3 ).
Description. Carapace broader than long, width 1.2–1.3 × length (n = 13), regions indistinct ( Fig. 1 View FIGURE 1 ). Dorsal surface smooth, finely pitted, convex ( Fig. 1 View FIGURE 1 ). Front deflexed, margin ridged in dorsal view ( Fig. 1 View FIGURE 1 ). Epigastric cristae smooth, very low and almost indistinct, separated from each other by narrow gap ( Figs. 1 View FIGURE 1 , 2A View FIGURE 2 ). Postorbital cristae smooth, very low, laterally extended, almost fused with epigastric cristae and epibranchial teeth ( Fig. 1 View FIGURE 1 ). Branchial regions inflated; cervical grooves very shallow, inconspicuous; mesogastric region convex ( Fig. 1 View FIGURE 1 ). External orbital teeth blunt, triangular with gently convex outer margins, each separated from anterolateral margin by small gap ( Figs. 1 View FIGURE 1 , 2A View FIGURE 2 ). Epibranchial teeth very small and inconspicuous ( Figs. 1 View FIGURE 1 , 2A View FIGURE 2 ). Anterolateral margins smooth, lined with numerous indistinct granules ( Fig. 1 View FIGURE 1 ). Posterolateral surfaces smooth ( Fig. 1 View FIGURE 1 ). Orbits large, supraorbital and infraorbital margins ridged ( Figs. 1 View FIGURE 1 , 2A View FIGURE 2 ). Sub-orbital, sub-hepatic and pterygostomial regions clearly divided by sutures, with smooth and pitted surface ( Fig. 2A View FIGURE 2 ). Epistome median lobe broadly triangular, lateral margins almost straight ( Fig. 2A View FIGURE 2 ).
Maxilliped III with merus subtrapezoidal, about as wide as long, median depression distinct; ischium subtrapezoidal, width about 0.9 × length, with distinct median sulcus, with anterior mesial margin rounded; exopod reaching to proximal one-fifth of merus, flagellum absent ( Fig. 3A View FIGURE 3 ).
Chelipeds (pereiopod I) unequal, relatively less inflated in females ( Figs. 1 View FIGURE 1 , 3F–I View FIGURE 3 ). Merus trigonal in cross section, margins weakly crenulated, surfaces generally smooth ( Figs. 1 View FIGURE 1 , 2A View FIGURE 2 ). Carpus with long, acute spine at innerdistal angle, spinule at base, surfaces generally smooth ( Fig. 1 View FIGURE 1 ). Major cheliped palm length about 1.1–1.2 × height in males (n = 5), 1. 2–1.3 × in females (n = 5); dactylus 1.0–1.1 × palm length in males (n = 5) and females (n = 5) ( Fig. 3F–I View FIGURE 3 ). Palm surface generally smooth, pitted, inner posterior region slightly granulated. Dactylus curved, as long as pollex. Occlusal margin of fingers lined with blunt, round teeth; small gape when finger tips in contact ( Fig. 3F–I View FIGURE 3 ).
Ambulatory legs (pereiopods II–V) slender, with short and sparse setae. Pereiopod III merus 0.6–0.7 × carapace length in males (n = 5), 0.6–0.7 in females (n = 5). Pereiopods V propodus 2.3–2.4 × as long as broad in males (n = 5), 2.3–2.5 in female (n = 5), shorter than dactylus ( Fig. 1 View FIGURE 1 ).
Male thoracic sternum generally smooth, pitted; sternites I–IV relatively narrow, width 1.6 × as length ( Fig. 2B, C View FIGURE 2 ). Sternites I, II separated by ridge, fused as broadly triangular structure; sternites II, III separated by conspicuous transverse sulcus, reaching edge of sternum; sternites III, IV fused, without visible demarcation ( Fig. 2B, C View FIGURE 2 ). Male sterno-pleonal cavity reaching anteriorly beyond level of posterior articular condyle of cheliped coxa ( Fig. 2B–D View FIGURE 2 ); median longitudinal groove separating sternites VII, VIII deep ( Fig. 2D View FIGURE 2 ). Male pleonal locking tubercles positioned at mid-length of sternite V ( Fig. 2D View FIGURE 2 ). Adult female vulvae ovate, relatively large but not reaching sternites V or VII, positioned closely to one another, orientation oblique to longitudinal axis of sterno-pleonal cavity ( Fig. 2F View FIGURE 2 ).
Pleon and telson triangular in males ( Fig. 2C View FIGURE 2 ) and broadly ovate in females ( Fig. 2E View FIGURE 2 ). Male pleonites III–VI progressively narrower, lateral margins nearly straight; pleonite VI 2.2 × as broad as long. Male telson 1.3 × as broad as long, with blunt apex ( Fig. 2C View FIGURE 2 ).
G1 slender, reaching beyond pleonal locking tubercle almost up to suture between sternites IV/V in situ ( Fig. 2D View FIGURE 2 ). G1 subterminal segment 2.6–2.7 × as long as terminal segment (n=3), tapering distally. G1 terminal segment relatively small, inverted foot-shaped; inner proximal margin strongly concave; inner distal margin almost straight to gently convex; apex acute, directed outward, orientation oblique to longitudinal axis of G1 ( Figs. 3C–E View FIGURE 3 , 7I, J View FIGURE 7 ). G2 subterminal segment 2.0–2.1 × as long as flagellum-like terminal segment (n=3) ( Fig. 3B View FIGURE 3 ).
Etymology. The specific name “incendium” means fire in Latin, which describes the live colouration of the species. The name thus is to be conceived as a noun in the nominative singular standing in apposition to the generic name.
Colour in life. Carapace, ambulatory legs and upper half of chelipeds generally bright red to orange, lower half of chelipeds white.
Habitat. Nanhaipotamon incendium n. sp. is a semiterrestrial species that burrows in the forest floor of relatively high-altitude rainforests at around 700 m a.s.l., which is the highest recorded for this genus ( Dai 1997, 1999). During summer, the rainforest not only receives a lot of rain but also abundant mist, which enables the crabs to freely roam on the forest floor. Crabs were collected from moist shallow burrows that do not reach the water table up to 50 m away from the nearest hillstream. In the winter when it is drier, however, the crabs burrow deep until they reach the water table. The once occupied shallow holes were found to be abandoned. Holes with fresh wet mud near the entrance were inaccessible to us due to the presence of roots and rocks, which made excavation nearly impossible. Immediately next to the hillstream, however, we found a few juveniles and a freshly molted adult male in mud holes under rocks. Longpotamon anyuanense (Dai, Zhou & Peng, 1995) is a large aquatic species, which lives under rocks in the hillstreams and is sympatric with the new species. Nanhaipotamon aff. aculatum were found from the same mountain, but at lower altitudes typically around 100 m a.s.l. Only on one instance, the habitats of the two species seemed to overlap to some extent as they were found side by side in 500 m a.s.l. (W.-H. Wang, pers. comm.).
Remarks. The overall intraspecific morophological variation is low. Larger specimens of N. incendium n. sp. tend to have a relatively wider carapace. The general shape of the G1 terminal segment in the new species seems to be stable, however, the inner distal margin varies from almost straight to gently convex ( Fig. 3C–E View FIGURE 3 ). Nanhaipotamon incendium n. sp. is a unique species within this genus in that it has smooth sub-orbital, sub-hepatic and pterygostomial regions ( Fig. 2A View FIGURE 2 ) [vs. granulated or striated in all other congeners ( Fig. 5A View FIGURE 5 ; Huang et al. 2018 b: figs. 3B, 8B)], and the exopod of the maxilliped III is relatively shorter and completely lacks a flagellum ( Fig. 3A View FIGURE 3 ) [vs. relatively longer exopod with flagellum in all other congeners ( Fig. 6A View FIGURE 6 ; Huang et al. 2018 b: fig. 5A)]. The relatively small G1 terminal segment of the new species is also unique within the genus ( Figs. 3C–E View FIGURE 3 , 7I, J View FIGURE 7 ) [vs. G1 terminal segment relatively large to very large in congeners ( Figs. 6C–E View FIGURE 6 , 7A–H View FIGURE 7 ; Huang et al. 2018 b: figs. 5C–E, 7)]. The G1 of the new species is very distinct from the sympatric Nanhaipotamon aff. aculatum due to the same reason ( Fig. 7D View FIGURE 7 ). In the field, the new species can readily be distinguished from the sympatric congener by its bright red to orange colouration ( Fig. 8A View FIGURE 8 ) (vs. blue or light brown carapace in N. aff. aculatum ; Fig. 8B View FIGURE 8 ). Nanhaipotamon cf. hongkongense from neighboring Shenzhen ( Fig. 7E–G View FIGURE 7 ) can also be bright orange to red and may look similar to the new species, but the smoothness of the carapace frontal regions immediately separates the two species apart.
Conservation status. Nanhaipotamon incendium n. sp. is only known from a single collection point and is likely highly endemic. We do not know of any current threats to this species, though its bright colours make it a prime target for the pet trade. As the collection of freshwater crabs are not yet regulated by law in China, we choose to remain discreet about the exact locality of the new species.
| AM |
Australian Museum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Potamiscinae |
|
Genus |