Auriculostoma lobata, Hernández-Mena, David Iván, Lynggaard, Christina, Mendoza-Garfias, Berenit & León, Gerardo Pérez-Ponce De, 2016
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4196.2.5 |
|
publication LSID |
lsid:zoobank.org:pub:87539887-8919-45C1-9326-EACB8B9283D9 |
|
DOI |
https://doi.org/10.5281/zenodo.6087433 |
|
persistent identifier |
https://treatment.plazi.org/id/A1247D4E-765D-FF9D-7DD6-72F9C32DF860 |
|
treatment provided by |
Plazi |
|
scientific name |
Auriculostoma lobata |
| status |
sp. nov. |
Auriculostoma lobata View in CoL n. sp.
( Figs. 1–2 View FIGURE 1 View FIGURE 2 )
Synonym. Auriculostoma astyanace of Salgado-Maldonado et al. (2011)
Type-host. machaca, Brycon guatemalensis Regan (Actinopterygii: Bryconidae ).
Type-locality. El Mangal Lagoon, Usumacinta River basin, Tenosique, Tabasco, Mexico (17°38'46.99" N, 91°22'58.0" W). GoogleMaps
Other localities. Lacantun River, Chiapas, Mexico (16°06′03″ N, 90°57′30″ W and 16° 14′ 46″ N, 90° 50′ 08″ W) GoogleMaps ; Usumacinta River , Chiapas, Mexico (16°49′24″ N, 90°53′18″ W) GoogleMaps .
Site of infection. Intestine.
Specimens deposited. Holotype (CNHE 1081); paratypes: 8 specimens from Tabasco ( CNHE 1082 ) and 6 specimens from Chiapas ( CNHE 7505 ) .
Etymology. The specific name lobata is the nominative feminine singular of the Latin adjective "lobatus" and is referring to the lobated form of the testes, in this case with 5 to 6 deep lobes.
Description. (Based on 15 adult specimens, nine from Tabasco, and six from Chiapas—measurements in Table 2). Body elongate, slightly narrowing at pharyngeal level. Tegument unspined. Oral sucker subterminal, funnel-shaped, well-developed, with 19 dome-like papillae arranged in 4 rows: 4 apical, 6 anterior (slightly noticeable), 4 on inner surface (two near mouth), and 5 on outer surface ( Fig. 2 View FIGURE 2 B,D). Oral sucker with a single pair of muscular lobes located on either side of oral sucker, with a broad base. Muscular lobes stretching from ventrolateral to dorsolateral side and slightly differentiated of apical region; dorsolateral side of lobes narrow with triangle-shaped “free” ends. Mouth subterminal. Prepharynx absent. Pharynx muscular, ovoid and well developed. Esophagus curved. Intestinal cecal bifurcation between pharynx and ventral sucker. Intestinal ceca long and narrow, reaching near to posterior extremity. Ventral sucker spherical, strongly muscular, and slightly larger than oral sucker. Testes in tandem, multilobated (deeply lobed), in posterior half of body. Anterior testis usually with 5 lobes and posterior testis with 6 lobes. Post-testicular space representing 21.4–29.4% (24.5%) of body length. Cirrus sac very long, forming several loops (3 to 5), extending to surpass the posterior border of ovary, containing elongate internal seminal vesicle, pars prostatica (bulb shaped), and wide muscular ejaculatory duct. Genital pore median, opening on ventral surface, between intestinal bifurcation and anterior margin of ventral sucker. Ovary pre-testicular, pre-equatorial, sinistral or dextral, oval and entire. Seminal receptacle saccate, post-ovarian. Laurer’s canal not observed. Mehlis’ gland posterior, between ovary and anterior portion of seminal receptacle. Vitelline follicles small, numerous, forming intra- and extracecal lateral fields along body, extending from cecal bifurcation to posterior end of body, and confluent at post-testicular area. Vitelline reservoir ventral to Mehlis’ gland. Uterus completely pre-testicular, intercecal, between anterior testis and genital pore. Eggs moderately small. Excretory vesicle I-shaped, reaching to posterior margin of posterior testis. Excretory pore terminal.
Remarks. Auriculostoma lobata n. sp. conforms to the diagnosis of Auriculostoma as recently amended by Razo-Mendivil et al. (2014), including the presence of a single pair of muscular lobes on either side of the oral sucker. The new species most closely resembles the type species of the genus, A. astyanace as they have similar morphological characteristics such as testes in tandem, a genital pore located between the intestinal bifurcation and ventral sucker, a curved esophagus, vitelline follicles extending from the cecal bifurcation to the posterior end of the hindbody, a cirrus sac forming several loops (up to 5), and an excretory pore terminal. However, they differ in that the new species possesses strongly lobated testes (anterior testis with 5 major lobes and posterior testis with 6 major lobes), and the cirrus sac extends to the post-ovarian region. Instead, A. astyanace possesses smooth (entire) testes, and cirrus sac does not surpass the ovarian region. Unfortunately, SEM microphotographs are not available for this species to be compared with those of the new species, and ultrastructure of the body surface may reveal additional differences between the two species.
The new species can be distinguished from the other congeners by the combination of some morphological characters. In particular, the new species differs from three of the other six congeneric species, i.e., A. diagonale , A. platense and A. totonacapanensis by having testes deeply lobed located in tandem rather than entire testes in oblique postion. Even though in some specimens of A. totonacapanensis testes seem to be in tandem (Razo- Mendivil et al. 2014), in most of them testes are oblique. Additionally, A. lobata n. sp. differs from A. diagonale and A. platense in the anterior extension of the vitelline follicles; in the new species, the vitelline follicles reach the cecal bifurcation, whereas in A. diagonale they reach the pharynx level and in A. platense follicles reach the midlevel of the esophagus. Auriculostoma lobata n. sp. differs further from A. totonacapanensis by having a genital pore located between the cecal bifurcation and the ventral sucker, and not at the level of the cecal bifurcation as in A. totonacapanensis . In addition, the ultrastructure of the body surface of both species provide additional information. We obtained SEM photographs of our specimens and compared them with those from Razo-Mendivil et al. (2014). Both species possess 19 dome-like papillae on the oral sucker surface arranged in 4 rows (4 apical, 6 anterior, 4 on inner surface, and 5 on the outer surface) and both have a single pair of muscular lobes located on either side of the oral sucker. However, they can be readily distinguished by the shape of the dorsolateral side of the oral lobes, which in the new species, are "free" and triangular-shaped (see Fig. 2 View FIGURE 2 B–C).
Finally, the new species is similar to the other three congeneric species, A. foliaceum , A. macrorchis and A. stenopteri by possessing multilobated testes. However, the new species is readily distinguished by the anterior extension of the vitelline follicles; in A. lobata n. sp., the vitelline follicles reach the cecal bifurcation, whereas in A. foliaceum , follicles are absent in the forebody while in A. macrorchis and A. stenopteri follicles extend up to the level of the oral sucker. Additionally, the new species differs from A. foliaceum by having a ventral sucker larger than the oral sucker, an excretory pore terminal, and a smaller oral sucker on average; in A. foliaceum the oral sucker is 1.5 times larger than the ventral sucker, the excretory pore is subterminal, and the oral sucker is larger. From A. macrorchis , the new species differs further because vitelline follicles are intra- and extracecal in the pretesticular region and not largely extracecal.
Character A. lobata n. sp. A. astyanace A. totonacapanensis A. diagonale A. foliaceum A. macrorchis A. platense A. stenopteri
* 14 5 11 2 1 1 1 2
BƟđy Ŀ 2,539P3,010 1,900P2,900 1,028P2,003 (1,402) 1,207P1,600 1,993 1,500 800 750P1,320
(2,735) (2,900)
BƟđy W 502P639 (581) 400P488 (488) 287P568 (398) 352P474 450 500 200 216P308
Ŀ 457P525 (477) (25P32%) (25) P (27P28% Ɵf bƟđy 430 (22% Ɵf bƟđy P P P
(16P19% (17) Ɵf bƟđy leŊǥth) leŊǥth) leŊǥth)
Ɵf bƟđy leŊǥth)
Ŀ 153P234 (204) 202P234 (234) 117P212 (156) 170P190 279 180 120 P
W (excluđiŊǥ 191P245 (206) 208P 246 (246) 128P223 (176) 170P208 374 200 P P đƟrsal lƟbes)
W (iŊcluđiŊǥ 243P335 (267) 256P304 (301) 121P 268 (214) P P P P P đƟrsal lƟbes)
Ŀ 114P139 (124) 93P115 (115) 39P92 (69) 45P57 95 70 P 60P96
W 82P112 (99) 90P118 (112) 44P101 (74) 63P68 100 50 P 60P88
Ŀ 71P141 (112) 70P141 (102) 71P96 (84) P 111 P P 40P80
Ŀ 238P319 (260) 250P285 (275) 153P240 (193) 213P221 207 150 120 148P190
W 233P324 (273) 250P330 (330) 154P259 (198) 213P227 202 P P 140P240
Ŀ: VS Ŀ 1:1.08P1.66 (1.29) 1:1.10P1.17 (1.13) 1:1.03P1.62 (1.25) 1: 1.16P1.25 1: 0.7 1: 0.83 1: 1 P
(1.21)
OS W: VS W (
exluđiŊǥ lƟbes) AT Ŀ 1:1.21P1.52 (1.34) 1:1.21P1.29 (1.23) 1:1.01P1.54 (1.13) 1:1.1P1.25 267P352 (312) 166P266 (256) 103P219 (149) 193P261 1:0.54 106 1:1P1.3 P 1:1 P 1:1.3 60P120 AT W 167P274 (206) 157P218 (218) 62P218 (130) 110P159 134 P P 15P72 PT Ŀ 318P396 (360) 186P349 (349) 118P259 (175) 221P304 167 P P 80P132 PT W 191P234 (215) 173P211 (205) 62P239 (130) 139P142 145 P P 80P100
……continued on the next page Character A. lobata n. sp. A. astyanace A. totonacapanensis A. diagonale A. foliaceum A. macrorchis A. platense A. stenopteri
OV Ŀ 164P217 (193) 160P230 (218) 93P210 (134) 156P161 145 P P 52P120
OV W 92P165 (120) 144P202 (182) 68P167 (107) 102P145 117 P P 32P52
SR Ŀ 150P201 (181) 157P 205 55P135 (84) 85P108 40 P P P
SR W 101P115 (115) 102P115 (115) 39P137 (75) 59P77 45 P P P Eǥǥs Ŀ 46P58 (52) 55P59 48P65 (54) 54P57 58P63 55 75 68P76
Eǥǥs W 23P35 (27) 34P41 30P41 (34) 28P32 28P29 29 39 28P40
RefereŊce This paper SchƟlz et al. 2004 RazƟPMeŊđivil et al. CurraŊ et al. 2011 CurraŊ et al. 2011 Sziđat 1954 Sziđat 1954 MaŊéPGarzỏŊ & 2014 GascỏŊ 1973
= FƟrebƟđy, OS= Ɵral sucker, PH = pharyŊx, ES= EsƟphaǥus, VS= veŊtral sucker, AT= aŊteriƟr testis, PT= PƟsteriƟr testis, OV= Ɵvary, SR= semiŊal receptacle, PSTS= PƟst‒testicular space, PCS= PƟst‒cecal space, GPD= ǥeŊital pƟre đistaŊce frƟm aŊteriƟr eŊđ Ɵf bƟđy. Ŀ = ĿeŊǥth, W = Wiđth. * Number Ɵf specimeŊs measuređ iŊ the ƟriǥiŊal đescriptiƟŊ.
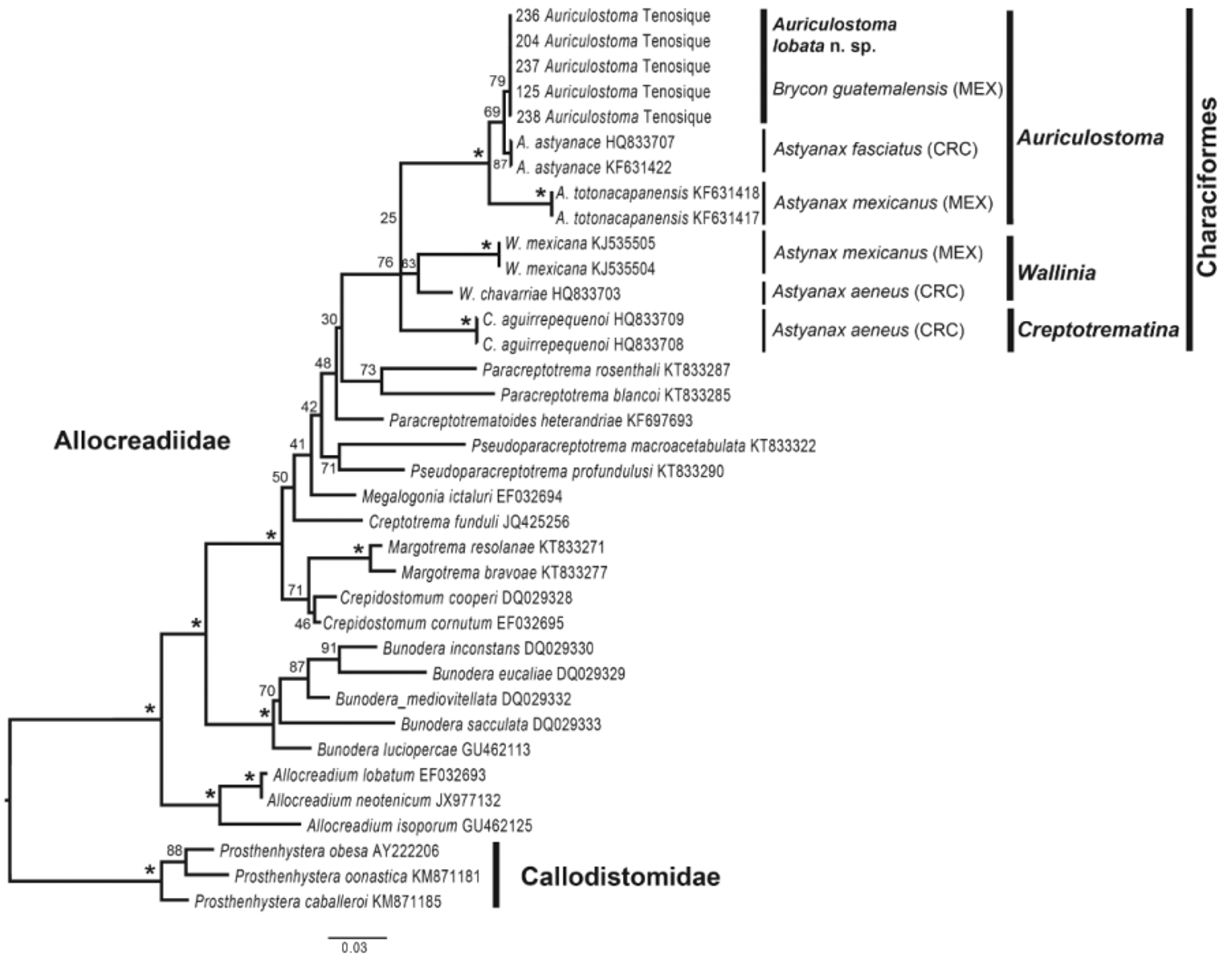
Phylogenetic analyses and molecular divergence. The final data set for the 28S rRNA gene consisted of 36 sequences, 33 of them representing 25 species of the Allocreadiidae and 3 species of the Callodistomidae as outgroups. Final alignment contained 1,776 base pairs. Nucleotide frequencies were A=0.213, C= 0.215, G= 0.312 and T= 0.261. The selected model for the ML analysis was GTR+GAMMA+I. ML yielded a single tree with ln= - 7038.128347. The ML phylogenetic tree showed an unresolved clade that contains the three genera of the Allocreadiidae commonly found as parasites of neotropical characids: Creptotrematina, Auriculostoma and Wallinia ( Fig. 3 View FIGURE 3 ). The phylogenetic analysis also showed that the genus Auriculostoma is monophyletic, with high bootstrap support value ( Fig. 3 View FIGURE 3 ). Each of the three congeneric species are recovered as monophyletic assemblages, with moderate bootstrap support. The five isolates of Auriculostoma lobata n. sp. are reciprocally monophyletic and are nested as the sister taxa of A. astyanace , and this clade as sister group of A. totonacapanensis . Intraspecific genetic divergence of the 28S rDNA sequences among individuals of A. lobata n. sp. was null. Genetic divergence between A. lobata n. sp. and A. astyanace , its sister species, was relatively low (0.29%), and between the new species and A. totonacapanensis reached 2.33%. As expected, intergeneric genetic divergence was higher. Between species of Auriculostoma and species of Creptotrematina , values ranged from 4.07 to 4.80%, and between species of Auriculostoma and species of Wallinia values varied between 2.47 and 5.38%.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |