Characella pachastrelloides ( Carter, 1876 )
|
publication ID |
https://doi.org/10.5281/zenodo.280590 |
|
DOI |
https://doi.org/10.5281/zenodo.6166229 |
|
persistent identifier |
https://treatment.plazi.org/id/A61787B7-DC6D-FFBC-FF33-FF5EFD11FB2C |
|
treatment provided by |
Plazi |
|
scientific name |
Characella pachastrelloides ( Carter, 1876 ) |
| status |
|
Characella pachastrelloides ( Carter, 1876)
( Figures 1 –3 View FIGURE 1 View FIGURE 2 View FIGURE 3. A , Table 2)
Synonymy.
Stelletta pachastrelloides Carter, 1876 : Carter 1876, p. 403.
Characella sollasi Topsent, 1890b : Topsent 1890b, p. 70; Topsent 1892, p. 40; Ferrer-Hernández 1914, p. 11.
Characella pachastrelloides: Sollas 1888 , p. 407; Topsent 1904, p. 96; Stephens 1915, p. 14; Topsent 1928, p. 133; Arnesen 1932, p. 13; Lévi & Vacelet 1958, p. 231; Boury-Esnault et al. 1994, p. 46; Maldonado 1996, p. 394; Maldonado 2002, p. 148; van Soest et al. 2007, Table 2; Cárdenas et al. 2011, Table S1.
Stryphnus pachastrelloides ( Schmidt, 1870) : Burton 1954, p. 220.
Not Ancorina pachastrelloides Schmidt, 1870 , p. 68 = Characella connectens Schmidt, 1870 according to Maldonado (2002).
Not Poecillastra sollasi ( Topsent, 1892) : van Soest & Stentoft 1988, p. 36 = Poecillastra sp. (this study).
Material. ZMBN 80248, Brattholmen, Hjeltefjord, western Norway, depth: 80-140 m, deep-water Lophelia pertusa reef, triangular dredge.
Comparative material examined.
Characella pachastrelloides , MNHN-DN22, Norman Collection, slide, Porcupine expedition; ZMAPOR 20375, Mingulay reef, Scotland, 56°49'27''N, 7°22'7''W, 128-137 m ( Fig. 2 View FIGURE 2 C, E, G); ZMBN 25629, 35º32'N, 07º07'W, Ibero-Moroccan Gulf, 1215 m ( Arnesen 1932) ( Fig. 2 View FIGURE 2 B, H); ZMAPOR 18041, Gulf of Cadiz, Spain, 742 m; ZMBN 85225, 38º16'N, 9º10'W, Setúbal canyon, South Portugal, 1451 m ( Fig. 2 View FIGURE 2 I); ZMBN 85241, Guilvinec Canyon, Bay of Biscay, 46°54'N, 05°19'W, 676- 691 m. Characella cf. pachastrelloides , MNHN-DCL3228, Manila, Philippines, 170-200 m, MUSORSTOM 1, St. 51; MNHN-DCL3229, slide, Manila, Philippines, 198 m, MUSORSTOM 2, St. 1.
Characella tripodaria ( Schmidt, 1868) , MNHN-DT756, holotype, Algeria.
Characella tuberosa Lévi, 1964 , MNHN-DCL1396, slide of holotype, 29°55'S, 31°20'E, Off Durban, South Africa, 430 m, Galathea expedition.
Poecillastra dilifera (de Laubenfels, 1934), MNHN-DNBE1, slide of paratype, Puerto Rico, 439- 548 m.
Poecillastra sp., ZMAPOR 5300, originally identified as Poecillastra sollasi (van Soest & Stentoft 1988), off Paynes Bay, Barbados, 216 m, det. P. Cárdenas.
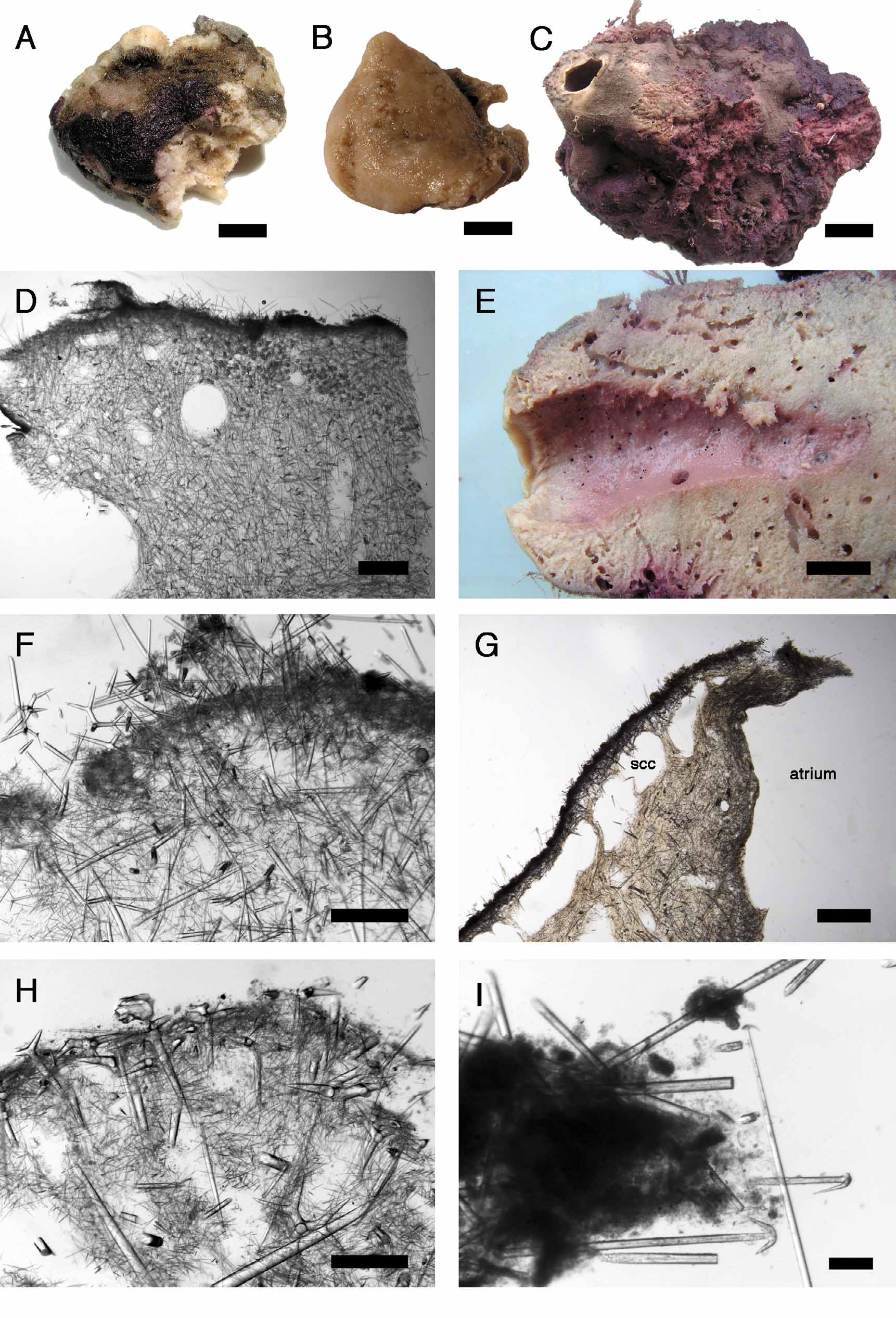
Outer morphology ( Fig. 1 View FIGURE 1 A). ZMBN 80248 is a massive fragment 5 cm long and 4 cm wide. Surface and choanosome color in ethanol is cream. The specimen is not compressible. Surface is irregular, strongly hispid and dirty (due to trapped sediments). The specimen is partly stained purple due to the encrusting sponge Hexadella dedritifera Topsent, 1913b which turns from bright yellow to purple in ethanol. There is no visible cortex. No oscules or pores were found.
Skeleton ( Fig. 1 View FIGURE 1 D, F). There is no real separated cortex, just a dense accumulation of paratangential microxeas II, reinforced by an underlying dense layer of paratangential microxeas I. This layer is 150–200 µm thick, thus invisible to the naked eye. Both orthotriaenes and dichotriaenes are present, more or less positioned radially with their cladomes tangential to the cortex. Some oxeas and triaenes cross the surface; they are responsible of the strong hispidation. Apart from the radial position of some triaenes, the arrangement of the spicules in the choanosome is confused. Amphiasters are commonly found in the cortex area but are also moderately present in the choanosome. Both kinds of microxeas are abundant in the choanosome, but the microxeas I are clearly predominant and form a dense meshwork. There is also a contamination of Geodia sterrasters in the choanosome.
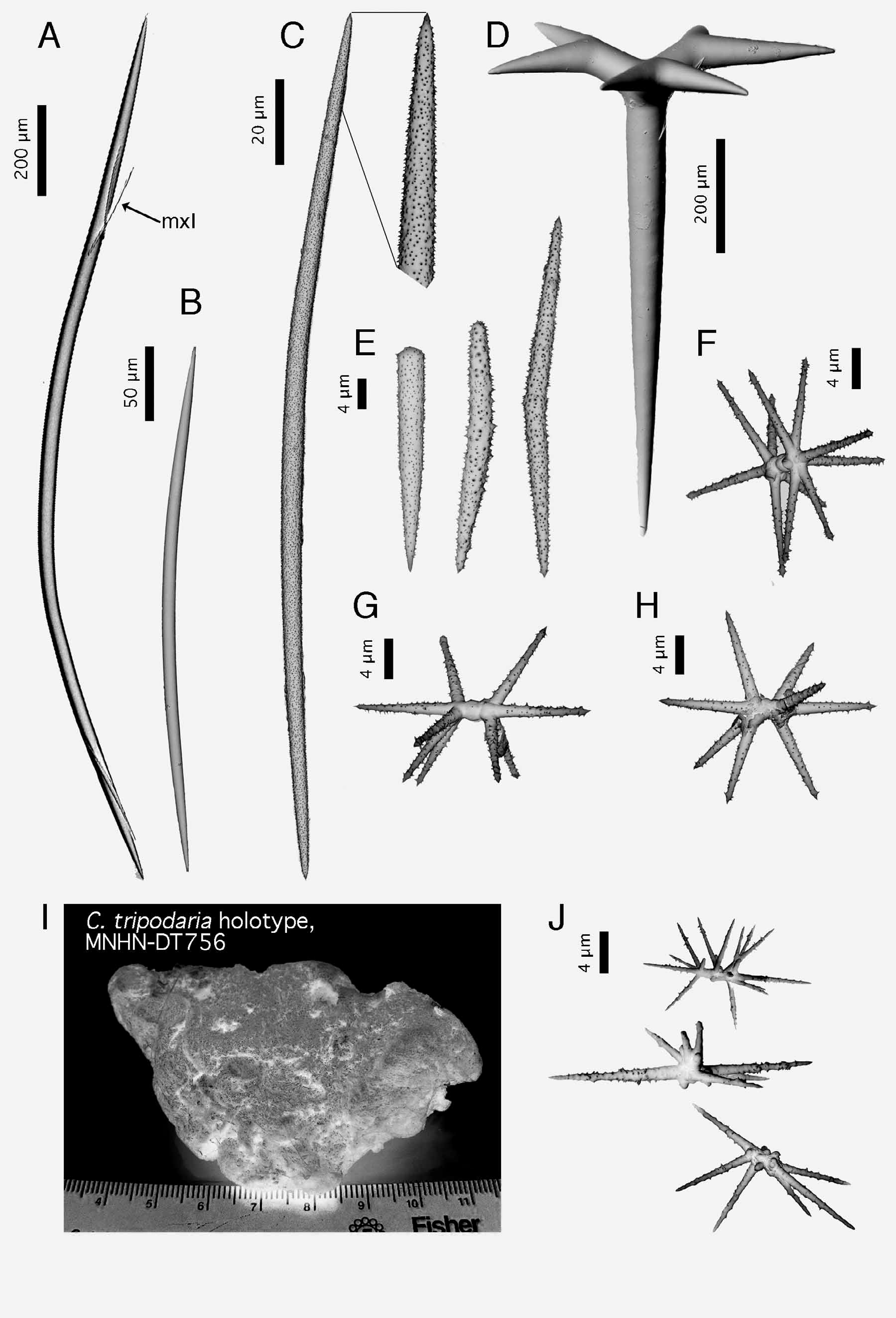
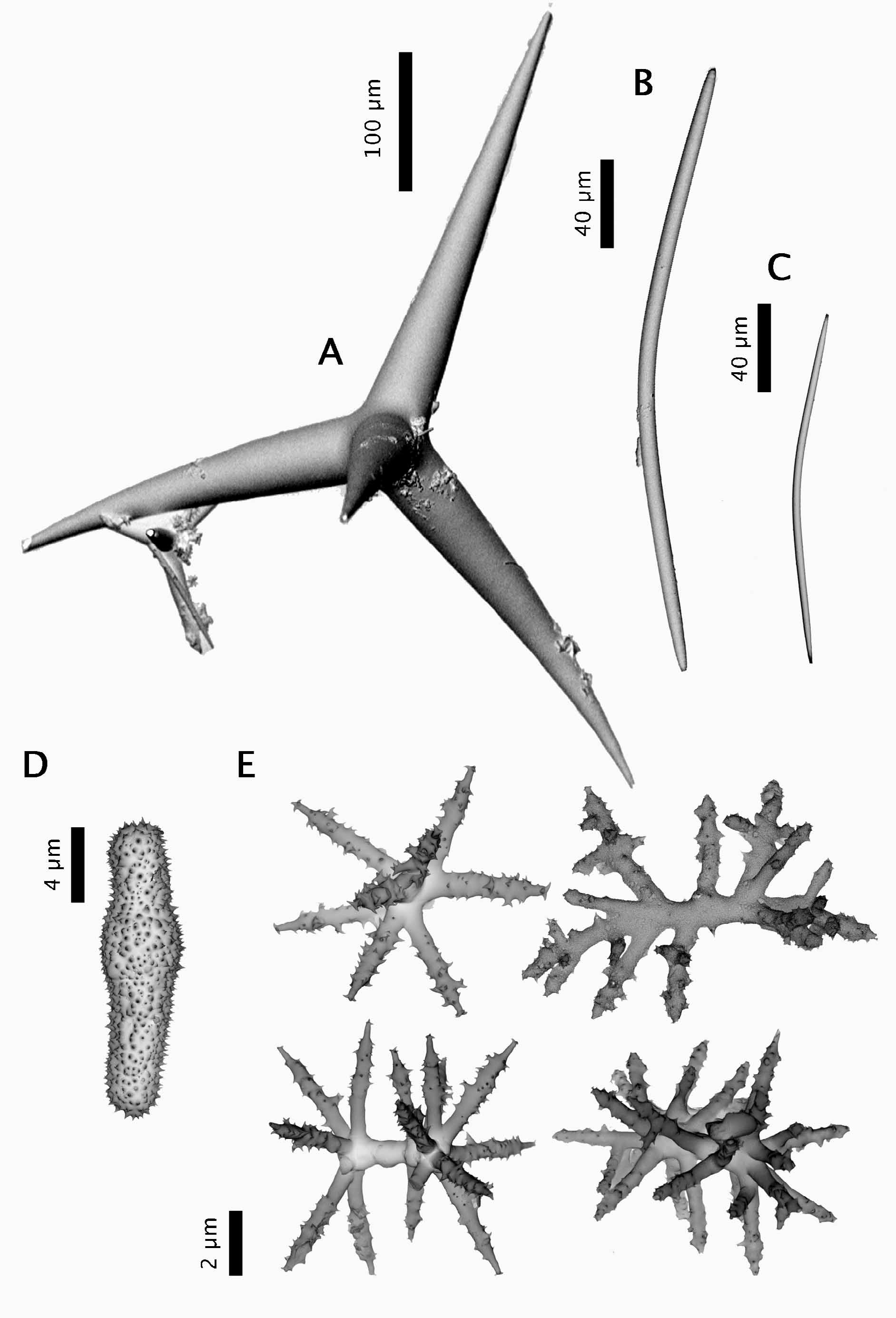
Spicules (ZMBN 80248) ( Fig. 2 View FIGURE 2 ). (a) oxeas I, stout, most are curved (once or twice), length: 2184- 1769 -1210 µm; width: 20- 37 -52 µm. (b) oxeas II, foreign?, slightly curved, smooth, sometimes modified to a style, rare, length: 300- 381.4 -438 µm (N=21); width: 9- 11.7 -13 µm (N=21). (c) ortho- and dichotriaenes, some with deformities such as irregular or additional clads, rhabdome length: 168- 408 -630 µm; rhabdome width: 11- 33 -49 µm; clad length for orthotriaenes: 83- 175 -316 µm (N=18); clad length for dichotriaenes: 70- 99 -129 µm + 72- 135 -194 µm (N=12). (d) amphiaster, 9-14 actines, microspiny, length: 13- 18 -29 µm. (e) microxea I, in high numbers, microspiny, usually straight but sometimes curved, sometimes modified to a microstyle, length: 80- 195 -259 µm; width: 2- 4.4 -5 µm. (f) microxea II, faintly microspiny, straight or bent, often centrotylotes, sometimes modified to a microstyle or a microstrongyle, length: 24- 35 -49 µm; width: 2- 2.6 -4 µm.
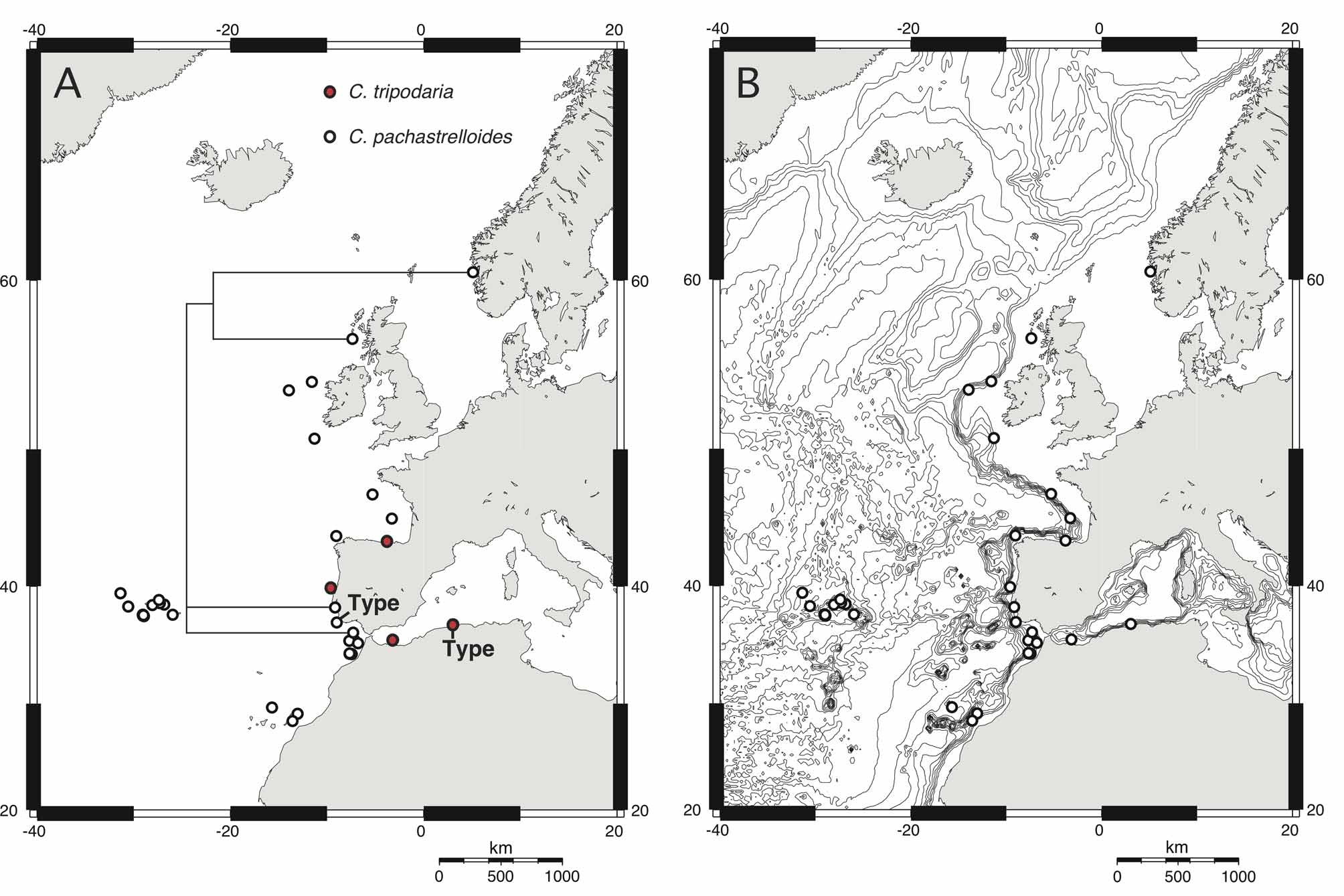
Distribution. ( Fig. 3 View FIGURE 3. A ). Norway (this study); Scotland (this study); Porcupine Bank (van Soest et al. 2007); Ireland ( Stephens 1915); Portugal ( Carter, 1876; Topsent, 1892; this study); Ibero-Moroccan Gulf ( Arnesen 1932; Boury-Esnault et al. 1994); Azores Islands ( Topsent 1904; 1928; Lévi & Vacelet 1958); Canary Islands ( Topsent 1928; Burton 1954);? Philippines ( Lévi & Lévi 1989).
Depth. 140 m- 1804 m ( Topsent 1928; this study).
Discussion. This is the first record of C. pachastrelloides in Norway, which extends its distribution range to the north ( Fig. 3 View FIGURE 3. A ). Our specimen was found in shallower waters than in previous records; we thus also stretch the depth range of that species. This should not come as a surprise since fjords are known to harbour deep-sea benthic organisms at shallower depths ( Strømgren 1970; 1971; Fosså et al. 2002). It should be noted that on the Norwegian coast, C. pachastrelloides can easily be confused with another Astrophorida : Stryphnus fortis Vosmaer, 1885 . They share a very similar massive morphology, with brownish hispid surfaces, large atria, and identical epibionts (e.g. encrusting sponges such as H. dedritifera ). However, S. fortis has a more conspicuous cortex with larger megascleres, sanidasters (8–12 µm) and large oxyasters (50–80 µm). Also, it does not possess microxeas.
Taking advantage that the Scottish specimen was nearly complete, we report here the first detailed observation of the oscule for this species. ZMAPOR 20375 was a massive lumpy specimen (17 cm high, 14 cm wide) ( Fig. 1 View FIGURE 1 C) which had a large oscule (ca. 2 cm in diameter) placed at its summit. It is similar to a Portuguese specimen described by Topsent (1892, pl. II, fig. 3). This oscule had a tough contractile membrane around it. Other smaller oscules (0.5–0.7 cm wide) were irregularly distributed. Exhalant openings are numerous in the 10 cm deep atrium ( Fig. 1 View FIGURE 1 E). A section in the tough contractile membrane showed that the structure of the cortex is identical to the rest of the specimen but that large sub-cortical canals appear ( Fig. 1 View FIGURE 1 G). We have observed such canals in the cortex outside the oscule, but never in such a regular pattern.
The intra-specific variability of the spicules observed during the last revision of C. pachastrelloides ( Maldonado 1996) is here confirmed ( Table 1 View TABLE 1 ). Oxeas II have never been observed in this species before. Due to their rarity, we cannot rule out the possibility that these Haplosclerida-like oxeas II could in fact be foreign spicules incorporated in the choanosome. This is supported by the numerous foreign Geodia sterrasters observed in our sections ( Fig. 1 View FIGURE 1 D). Only one oxea II was found in the Scottish specimen. On the other hand, these oxeas II might characterize a derived Norwegian population of C. pachastrelloides , but more specimens and genetic data are needed to conclude. Also, as noted before us, C. pachastrelloides can either have orthotriaenes, dichotriaenes, or both ( Topsent 1928; Maldonado 1996). When dichotriaenes are present, the protoclades are usually shorter than the deuteroclades. We also noticed that the morphology of the triaenes (length and width) could be quite variable between specimens ( Figs. 1 View FIGURE 1 F, H). For example, the rhabdome of the triaenes was quite short in our Norwegian specimen (mean of 480 µm) and quite long in the Ibero-Moroccan specimens (ca 770 µm). The different environment (shallower depth, colder water) of the Norwegian specimen may explain these differences.
Maldonado (1996) acknowledges the presence of anatriaenes in the holotype ( Carter 1876) and specimens from the Ibero-Moroccan Gulf ( Boury-Esnault et al. 1994), but remains uncertain whether these are exogenous elements incorporated from the sediment. However, these same anatriaenes have been found in other specimens from Ireland ( Stephens 1915), the Azores ( Lévi & Vacelet 1958) and in ZMBN 85225, our sample from Portugal ( Fig. 1 View FIGURE 1 I). In our opinion, anatriaenes were clearly not exogenous since thick sections of this specimen show them perpendicular to the sponge surface, with their cladome outside and their rhabdome crossing the cortex and the choanosome under it. They furthermore resemble the ones found in the holotype ( Maldonado 1996, Figure 8 View FIGURE 8 c). Actually, Topsent (1892) based his description of a new species from West Portugal ( Characella sollasi ) on the absence of anatriaenes and of oxyasters (both observed in the original description of C. pachastrelloides by Carter). After examining slides from the Porcupine expedition ( Carter 1876), Topsent (1904) later suggested that the anatriaenes and oxyasters from the holotype were probably exogenous and thus synonymized C. sollasi and C. pachastrelloides . He was right concerning the oxyasters but wrong concerning the anatriaenes. This further underlines the fact that depending on the specimens of C. pachastrelloides , anatriaenes can be absent or quite rare and thus easily overlooked. These were in fact not found in the Norwegian specimen.
This last result casts serious doubts on the validity of the other Characella species in the area: C. tripodaria . Records of this species are scarce ( Fig. 3A View FIGURE 3. A ) but we notice that its distribution clearly overlaps that of C. pachastrelloides . Spicule measurements of the holotype of C. tripodaria match those of C. pachastrelloides ( Table 1 View TABLE 1 ). The triaenes, apparently smaller in C. tripodaria , are in fact similar in size to those of the Norwegian specimen of C. pachastrelloides . Also, we found a second category of smooth oxeas while reexamining the holotype. Topsent (1938) had considered them to be a contamination from a Reniera but since they also match the oxeas II found in our Norwegian specimen, they might actually belong to C. tripodaria . At this moment, we are unsure on the status to give to these oxeas. According to Maldonado (1996), only three differences discriminate these two species: C. tripodaria has (i) anatriaenes, (ii) few amphiasters and (iii) amphiasters with numerous actines (although he does not give any specific range or number). We have therefore looked at the quantity and morphology of the amphiasters present in our anatriaenes-bearing Characella specimens. Amphiasters in some of the specimens examined were a bit less abundant; all these specimens came from the Gulf of Cadiz (ZMBN 25628, ZMAPOR 18041, Balgim specimen). But, the amphiasters of our specimens all had 7–11 actines, versus 5–18 in the holotype of C. tripodaria . On average, the amphiasters of the C. tripodaria (holotype) clearly had extra actines (or buds of actines) on the central shaft ( Fig. 2 View FIGURE 2 J). Underdeveloped actines were common, making the amphiasters very irregular ( Fig. 2 View FIGURE 2 J). Often, the central shaft was shortened or missing, giving these amphiasters the appearance of euasters ( Fig. 2 View FIGURE 2 J), which is what Carter (1876) may have observed. The amphiasters from the Alboran specimens of C. tripodaria had extra actines but no underdeveloped/irregular actines ( Maldonado, 1996). Conversely, C. pachastrelloides amphiasters never had extra actines on the shaft ( Fig. 2 View FIGURE 2 F–H), and they were all fairly regular (fully developed and always with a shaft). In our opinion, the irregular amphiasters with additional actines of C. tripodaria could be due to the environment and we therefore doubt the validity of C. tripodaria . However, consistent small differences have been shown to be of importance in sponge taxonomy and we therefore prefer to cautiously keep it as a valid species, until genetic data can settle the matter.
When we map the bathymetry over the distribution of these two species ( Fig. 3 View FIGURE 3. A B), we notice that the distribution of C. pachastrelloides and C. tripodaria perfectly follow the shelf break of the European and African continental margins. This also might explain why both species are often associated with deep-water corals ( Maldonado 1996; van Soest et al. 2007; this study).
28S sequences were identical for all the specimens sequenced. On the other hand, we found 1 bp. difference between the COI of C. pachastrelloides from Norway / Scotland and C. pachastrelloides from Portugal / Spain ( Fig. 1 View FIGURE 1 A). Over such a geographical distance and with such a small genetic difference, studies have shown that sponge species can be cryptic ( Cárdenas et al. 2007), but in our case, no clear morphological difference justifies the splitting of C. pachastrelloides into a northern and a southern NEA species.
= 30 unless stated otherwise between parentheses. - = not referred; n.f. = not found in this study.
Material Depth amphiaster Microxea I Microxea II Triaene Orthotriaenes (clads) or Oxeas I Oxeas II Anatriaenes (m) (length) (length/width) (length/width) rhabdome dichotriaenes (length/width) (length/width) (length/width/clads) (length/width) (protoclade +
deuteroclade)
Characella pachastrelloides 80-140 13- 18.1 -29 80- 194.9 -259/ 24- 35.2 -49/ 168- 407.8 -630/ 83- 175.1 -316 (18) 1210- 1769.1 - 300- 381.4 -438/ n.f. ZMBN 80248 * 2- 4.4 -5 2- 2.6 -4 11- 32.6 -49 (orthotriaenes) 2184/ 9 - 11.7 -13
Hjeltefjord, Norway 70- 98.8 -129 + 20- 37 -52 (21) 72- 135.6 -194 (12)
(dichotriaenes)
Cape St. Vincent, Portugal 683 13 245/ 46.5/ 850/ 490 3660-4620/ - 3660-6640/ holotype 6.4 8.5 70 (orthotriaenes) 84-100 21/
( Sollas 1888; Maldonado 1996) 100-170 (rare) West of Portugal 300-736 20 200-220/ 35-40/ - - 2500-3200/ - n.f. ( Topsent 1892) - - (orthotriaenes) -
ZMBN 85225 * 1393 13- 21.0 -39 155- 219.7 -287/ 20- 31.0 -39/ 540- 827.9 -1052/ 428- 721.4 -1026 Up to 3000/ n.f. 2750- 2901 -3052
Setúbal canyon, Portugal 4- 5.0 -6 3- 3.0 -3 51- 66.4 -81 (N=15) 30- 49.5 -85 (10) (2)/ (N=17) (orthotriaenes) 20- 27.4 -30 (10) / 61- 145.2 -204 (10) ZMBN 25629 * 1215 13- 16.4 -25 166- 242.9 -290/ 26- 33.5 -44/ 489- 769.7 -1020/ 102- 141.9 -193 + 1578- 2672.1 - n.f. n.f.
Ibero-Moroccan Gulf 5- 5.0 -7 90- 215.7 -306 3025/
( Arnesen 1932) 2- 2.0 -2 31- 62.4 -90 (dichotriaenes) 40- 70.4 -100
Ibero-Moroccan Gulf 948- 1515 12- 20 -25 175- 216 -260/ 32- 20 -50/ 580- 770 -950/ 480- 534 -560 2100- 2537 -2980/ - -/
( Boury-Esnault et al. 1994) (rare) 4- 4 -6 4-5 55 - 68 -75 (ortho- and 50- 61 -70 -/ dichotriaenes) 105-115 (rare) Azores Islands 523-845 (rare) - Up to 40/ Up to 500-800/ 110 + 230-280 3000-4000/ - n.f. ( Topsent 1904) - - (ortho- and -
dichotriaenes)
Azores Islands 370-460 13-14 110-275/ 20-25/ 550/ 550 1600-2750/ - -/
( Lévi & Vacelet 1958) (rare) 4-7 2-3 17 (orthotriaenes) 45 -/ 110 (rare) 170-500/ 1500-3000/ n.f. n.f.
Characella cf. pachastrelloides 170-520 25-30 90-210/ 25- 32.6 -38/ 200-600/ 20-70 25-100
Manila, Philippines 3-6 3- 4.1 -4.5 20-75 (orthotriaenes)
( Lévi & Lévi 1989) except * (10)*
Characella tripodaria ? 10- 18.4 -30.2 115-180/ 35-45/ 180-400/ 180-400 1000-1600/ 267- 343.6 -406*/ -/ BNHM:68:3:2:36 (15)* 1.5-2.5 1.5-2.5 15-22 (orthotriaenes) 10-40 5 - 7.0 -10* 10/ Algeria (few) foreign? 25 holotype
( Maldonado 1996) except *
Alboran Island 70-120 10-17 121-243/ 30-50/ 200-524/ 200-524 900-2430/ - 1300-3000/ ( Maldonado 1996) (rare) 3-5 2-4 25 -30 (orthotriaenes) 20-58 9 -19/ 20-75
spicules measured for this study.
We have re-examined the specimen identified as Poecillastra sollasi (ZMAPOR 5300) from the Barbados (van Soest & Stentoft 1988). It has a clear plate-like morphology, clearly different from the massive C. pachastrelloides . Its calthrop-like triaenes are significantly smaller than the NEA triaenes of C. pachastrelloides . Also, its microscleres are very different from C. pachastrelloides : (i) most of its microxeas are conspicuously centrotylote, (ii) the streptasters are mainly plesiasters (sometimes modified to amphiasters) and few metasters-spirasters, (iii) plesiasters are very large (diameters of 60 mm are common, and we observed sizes up to 100 mm). Poecillastra dilifera (holotype USNM 22331) from the Puerto-Rico trench has a very similar external morphology to the Barbados specimen. However, a comparison with a slide of the paratype of P. dilifera (MNHN-DNBE1) showed that P. dilifera had (i) only one size of microxea, of length 77.5- 178.3 -262 µm (vs. two sizes: 155–220 µm and 36– 60 µm in the Barbados specimens), (ii) much thicker microxeas, width 2- 4.8 -7.5 µm (vs. 2–5 µm), (iii) much more spirasters and (iv) smaller plesiasters, diameter of 23-38 mm. For these reasons, we temporarily consider the Barbados specimens different from P. dilifera . The Barbados specimens are atypical because they possess simultaneously Characella characters (two size categories of microxeas) and Poecillastra characters (spirasters and metasters, plate-like external morphology). Both genera have been shown to be phylogenetically quite distinct and it has been suggested to prioritize the morphology of the streptasters (amphiasters vs. spirasters-metastersplesiasters) to better separate these genera ( Cárdenas et al. 2011). Until a comprehensive revision of these genera is made, the Barbados species is named Poecillastra sp. We suspect this species to be new but a comprehensive revision of western Atlantic Poecillastra / Characella species is required, a task beyond the purpose of our study.
Characella tuberosa from Durban ( South Africa), synonymized with C. pachastrelloides by Lévi & Lévi (1989), is actually fairly different. C. tuberosa (MNHN-DCL 1396) had a few spirasters mixed with a majority of amphiasters. These spirasters are never present in C. pachastrelloides . We therefore propose to resurect C. tuberosa as a valid species until further specimens from South Africa can be examined. We also stress that this species should remain in the Characella genus since it has two sizes of microxeas and amphiasters as the main streptaster. Characella flexibilis Lévi, 1993 from New Caledonia is also fairly close to C. pachastrelloides but is discriminated by its cup/blade morphology and its elasticity ( Lévi 1993). On the other hand, we re-examined specimens and slides (MNHN-DCL3228–3229) of the C. pachastrelloides collected in Manila ( Philippines) ( Lévi & Lévi 1989). The only difference with our NEA specimens is slightly wider microxeas II: they were 3- 4.1 -4.5 µm (N=10, MNHN-DCL3229) whereas the ones we measured in the NEA specimens were on average thinner than 3 µm ( Table 2). Genetic data is now needed to investigate if this is a cryptic species, different from C. pachastrelloides , as suggested by its remote geographic location.
| ZMBN |
Museum of Zoology at the University of Bergen, Invertebrate Collection |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Characella pachastrelloides ( Carter, 1876 )
| Cárdenas, Paco & Rapp, Hans Tore 2012 |
Characella flexibilis Lévi, 1993
| Levi 1993 |
Characella tuberosa Lévi, 1964
| Levi 1964 |
Hexadella dedritifera
| Topsent 1913 |
Poecillastra sollasi (
| Topsent 1892 |
Characella sollasi
| Topsent 1890 |
Characella pachastrelloides:
| Sollas 1888 |
Stelletta pachastrelloides
| Carter 1876 |
Stryphnus pachastrelloides (
| Schmidt 1870 |
Ancorina pachastrelloides
| Schmidt 1870 |
Characella connectens
| Schmidt 1870 |
Characella tripodaria (
| Schmidt 1868 |