Hoperius planatus Fall, 1927
|
publication ID |
https://doi.org/ 10.1649/0010-065x-68.2.321 |
|
persistent identifier |
https://treatment.plazi.org/id/A61AA54C-FFCD-127A-4E04-0FBE4DF6F5E4 |
|
treatment provided by |
Valdenar |
|
scientific name |
Hoperius planatus Fall, 1927 |
| status |
|
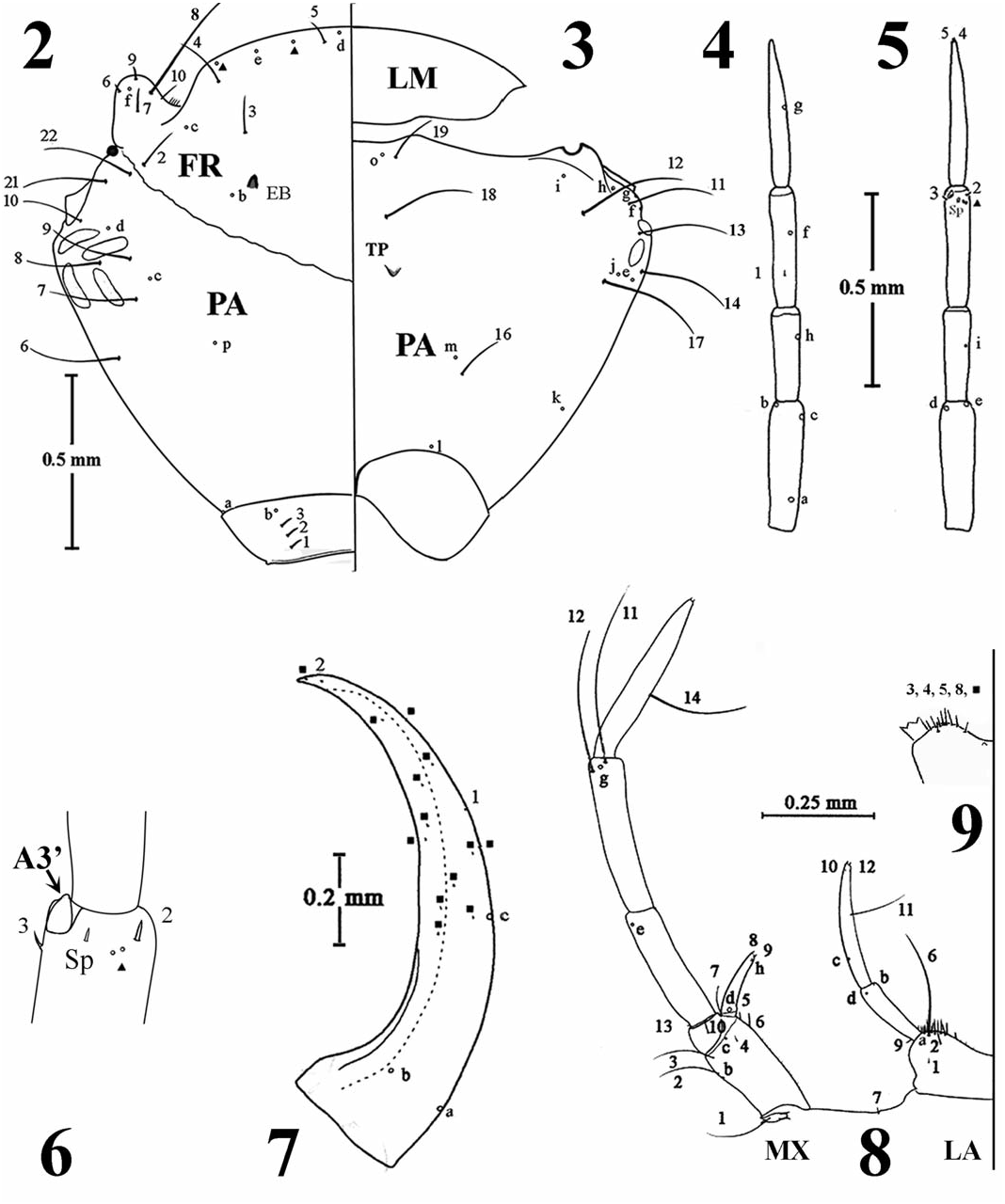
Hoperius planatus Fall, 1927 View in CoL ( Fig. 8 View Figs ): Cardo reduced; stipes prominent, sub-
cylindrical with length exceeding width, lateral
first instar
(Figs. 1–15) and medial margins somewhat parallel, medial
surface spinulate; lacinia absent; galea promi-
Diagnosis. The first instar of H. planatus can nent, subconical, curved inward, length slightly be distinguished from those of other Colymbetini over half that of palpomere (MP) 1, palpus length, by its restricted geographic distribution and the excluding prominent palpifer, 1.06–1.14 mm, following combination of characters: cranial dorso- MP1 0.30–0.35 mm, MP2 0.35–0.36 mm, MP3 ventral silhouette approximating that of an irregu- 0.41–0.43 mm. Labium ( Figs. 8, 9 View Figs ): Prementum lar convex hexagon; limited lateral expansion of approximately twice as wide as long, strongly temporal regions ( Figs. 2, 3 View Figs ); frontoclypeus with emarginated medially, small relative to maxilla and mandible, lateral margin distant from medial to the combined length of meso- and metaterga, margin of stipes; surface with densely distributed meso- and metaterga subequal in length; protergum microspinulae; postmentum, short with an expan- with posterior transverse carina, continuous latersive base; palpus 0.43–0.48 mm, palpomere (LP) ally; meso- and metaterga with anterior and pos- 1 0.17–0.19 mm, LP2 0.26–0.29 mm. Thorax: terior carinae, posterior carinae continuous laterally; Ecdysial suture present, protergal length subequal protergum with hair-like marginal and interior setae,
325 sparse hair-like setae on posterior and lateral mar- ments of pro-, meso-, and metathoracic legs: coxae gins of meso- and metaterga; minute densesly dis- 0.81–0.86, 0.84–0.89, 0.85–0.90 mm; trochanters tributed mirospinulae on terga; spiracles absent. 0.35–0.36, 0.36–0.42, 0.38–0.42 mm; femora
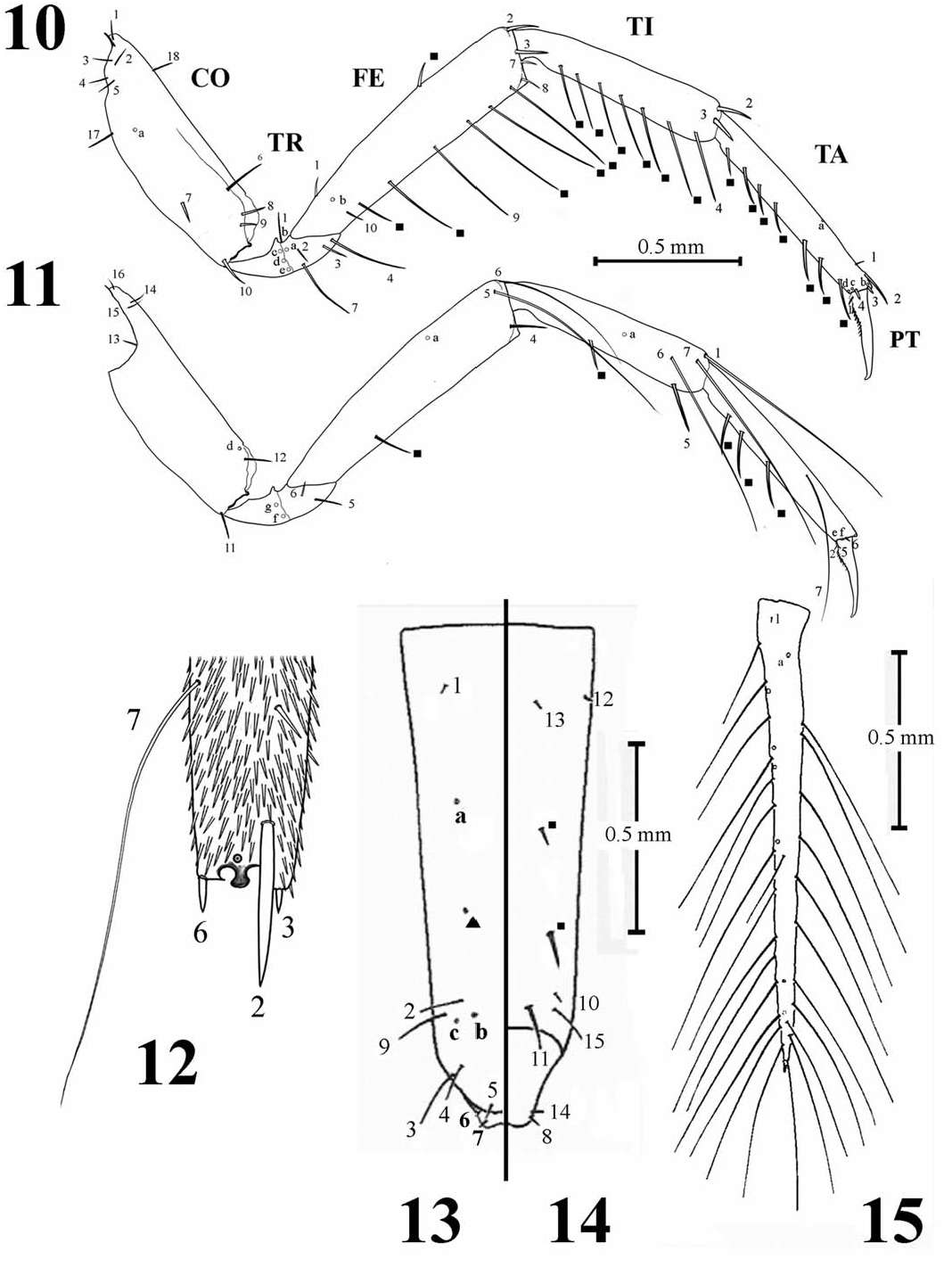
Legs ( Figs. 10–12 View Figs ): Natatory sensilla absent; series 0.88–0.93, 0.96–1.02, 1.04–1.08 mm; tibiae of ventral spinulae well-developed on pro-tibia and 0.50–0.56, 0.62–0.70, 0.78–0.86 mm; tarsi tarsus only; numerous small spinulae on surface 0.52–0.55, 0.64–0.67, 0.78–0.84 mm; anterior/ of all leg segments ( Fig. 12 View Figs ), numbers increasing posterior tarsal claws on pro- (0.52–0.53/0.64– ventrally on distal segments; abbreviated coxal 0.68 mm), meso- (0.67–0.69/ 0.59–0.63 mm), and sutures present; trochanter divided into 2 regions metathoracic legs (0.74–0.77/ 0.74–0.78 mm); (1 Tr; 2 Tr); respective lengths of individual seg- each claw with ventroproximal spinulae. Abdomen:
327
Terga with densely distributed microspinulae, seg- and 18–19 additional lateral, medial, and dorsal ments 1–7 lacking spiracles; segments 1–6 strongly spine-like and/or hair-like setae.
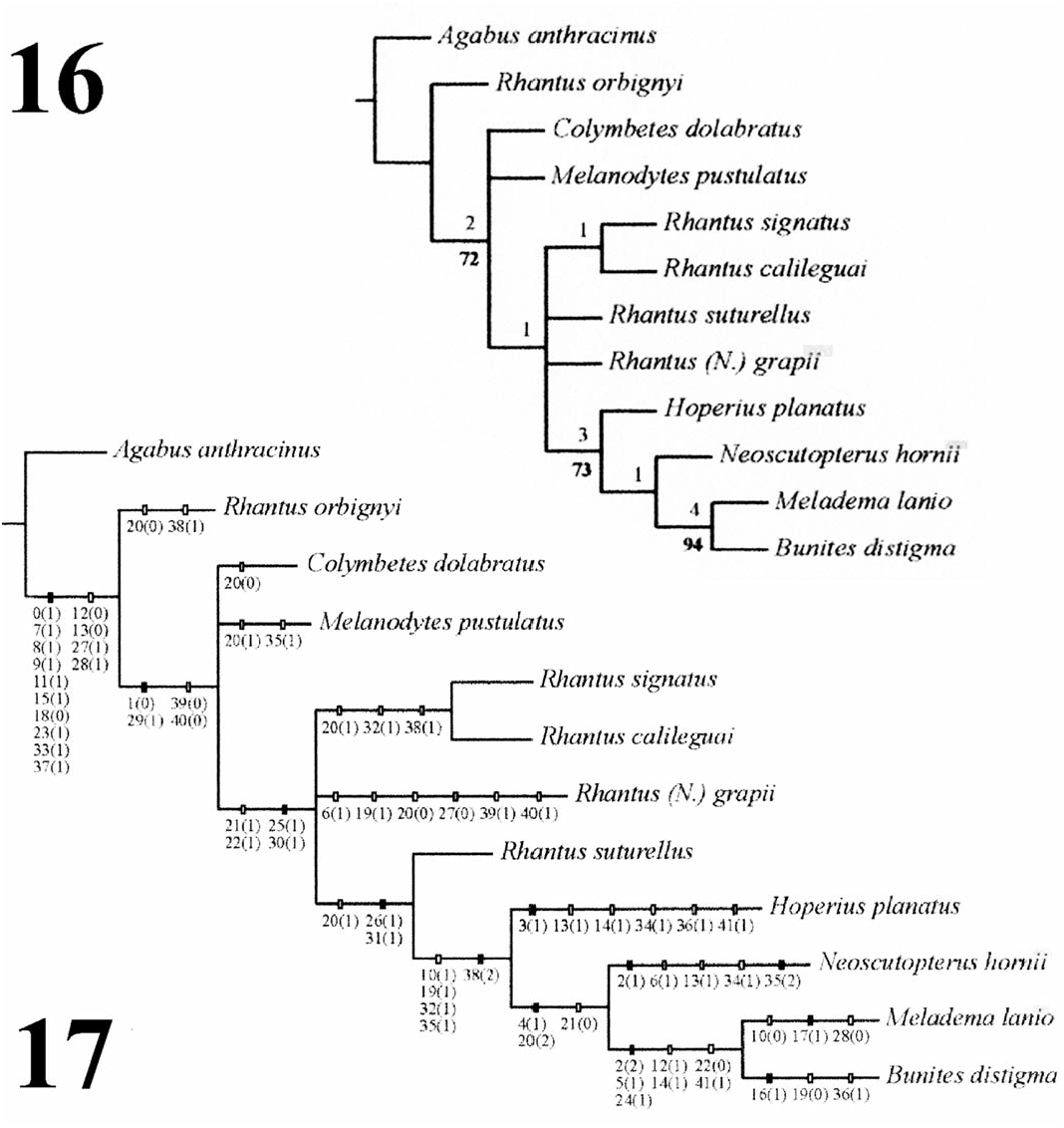
sclerotized dorsally with anterior and posterior Character Analysis. Forty-two first-instar charcarinae, posterior carinae continuous laterally; acters were included in the phylogenetic analysis, membranous laterally and ventrally, terga with setae with 38 coded as binary and four as multistate of variable lengths, primarily along posterior carinae, ( Table 1). The analysis of the data matrix ( Table 2) segments 4–6 with prominent ventral setae; seg- using TNT resulted in four most parsimonious ments 7–8 completely sclerotized, segment 7 with trees of 67 steps (CI = 0.69; RI = 0.70). These prominent posterior dorsal and ventral setae; dorsal equally parsimonious trees differed in the relative length of segment 8 ( Figs. 13, 14 View Figs ) 1.28–1.37 mm, positions of Colymbetes , Melanodytes , and some with siphon length 0.30–0.32 mm. Urogomphus Rhantus species. For this reason, the strict consen- ( Fig. 15 View Figs ): One-segmented, length 1.32–1.42 mm, sus was calculated, in which the genus Hoperius densely covered with microspinulae. Chaetotaxy was recovered as sister to a clade formed by the ( Figs. 2–15 View Figs View Figs ; Tables 1, 2): Notable similarities with genera Neoscutopterus , Meladema , and Bunites and variation from the chaetotaxy of A. anthracinus ( Fig. 16 View Figs ). Characters were mapped in one of the (Alarie 1995, 1998) and representatives of most parsimonious cladograms ( Fig. 17 View Figs ).
Colymbetini ( Table 2) include: Frontoclypeus Bionomics. First instars and concurrent second with FRe present, 2 additional submarginal and third instars of H. planatus were from a lower pores ( Fig. 2 View Figs ); antennomere A3 with an additional Piedmont ephemeral back-pool habitat formed in ventroapical spinula and 2 ventroapical pores the channel of Town Creek in Baldwin Co., Georgia. ( Fig. 6 View Figs ); mandible with about 10 additional minute These larvae were the only dytiscids collected. setae ( Fig. 7 View Figs ); maxilla with MXa absent; labium Back-pool habitats are typically flooded only during with prementum with 8–9 relatively elongate periods of heavier precipitation in the drainage spine-like anterodorsal setae (LA3, LA4, LA5, basin of the creek and are intermittently dry, or nearly LA8 + 4–5 additional setae) ( Fig. 9 View Figs ), LP2 with so, on one or more occasions annually. Production 2 additional minute, dorsoapical setae near LA 10 in these types of systems is typically supported by and LA12; femora with FE5 and FE6 elongate allochthonous detrital material.
and hair-like, additional setae of 1 dorsal, 1
posteroventral, 3–5 anteroventrals; tibiae with TI6 DISCUSSION
and TI7 elongate and hair-like, additional setae of
6–7 anteroventrals and 2–3 posteroventrals; tarsi The presence of third instars, as well as a third with TA1 small with subapical anterodorsal origin, instar collected from a lower Piedmont marsh TA2 anterodorsal distal and spiniform, TA7 elon- (Barman et al. 1996), indicates that oviposition gate hair-like with proximal posterodorsal origin by H. planatus in this area would have begun ( Figs. 10–12 View Figs ), additional setae of 6–8 antero- at least in January, assuming a Type I life cycle ventrals and 3 posteroventrals. Abdominal segment ( Nilsson 1986) with developmental rates commen- 8 with 1–2 asymmetrically distributed additional surate with other medium-sized dytiscids (Barman setae ( Fig. 14 View Figs ); urogomphus with UR1 and URa 1996). Precipitation rates in the area increase from present, identification of URb and URc prevented December through March (Plummer 1983), restorby 4–6 additional pores, one minute subapical seta ing and/or producing and maintaining numerous
Characters
Taxa 00–04 05–09 10–14 15–19 20–24 25–29 30–34 35–39 40–41 Agabus anthracinus 01000 00000 00110 00010?0000 00000 00000 00001 10 Bunites distigma 10201 10111 11101 11000 20011 11111 11110 1112??1 Colymbetes dolabratus 10000 00111 01000 10000 00010 00111 00010 00100 00 Hoperius planatus 10010 00111 11011 10001 11110 11111 11111 1112??1 Meladema lanio 10201 10111 01101 10101 20011 11?01 11110 1012??1 Melanodytes pustulatus 10000 0??1? ?? 000 10000 10010 00111 00010 1010? ?? Neoscutopterus hornii 10101 01111 11010 10001 20110 11111 11111 2012??0 Rhantus calileguai 10000 00111 01000 10000 11110 10111 10110 00110 00 Rhantus (Nartus) grapii 10000 01111 01000 10001 01110 10011 1?010 00101 10 Rhantus orbignyi 11000 00111 01000 10000 00010 00110 00010 00111 10 Rhantus signatus 10000 00111 01000 10000 11110 10111 10110 00110 00 Rhantus suturellus 10000 00111 01000 10000 11110 11111 11010 00100 00
highly productive temporary pools. Since average 1832), Agabus disintegratus (Crotch, 1873) and January air temperature for the central lower Agabus punctatus Melsheimer, 1844 .
Piedmont is about 7 °C (Anonymous 2013), there The first instar of H. planatus is characterized by is neither substantive nor persistent ice-cover to the presence of additional pores on the frontoclypeus deter exploitation of these systems by dytiscids (character 3, Fig. 2 View Figs ), representing a unique char- and dytiscid prey species. The limited cold tolerance acter state within the Colymbetini . Reduction in indicated for H. planatus that permits oviposition the overall size of the prementum (most evident and larval development during central Georgia’ s in the reduced length of the palpi as compared to relatively mild winters has also been observed that of the maxillary palpus) and in the length (Barman et al. 1996) for other dytiscids (e.g., (height) of the postmentum ( Fig. 8 View Figs ) is also impor- Hydroporus signatus Mannerheim, 1853 , Neoporus tant. These traits along with the presence in later undulatus (Say, 1823), Platambus stagninus (Say, instars of a shorter antennomere 2 and of a variable 329
number of secondary setae on antennomeres 1 and Investigaciones Científicas y Técnicas and proj- 2 and maxillary palpomere 1 (Alarie and Hughes ect PICT-2010-0526 from Agencia Nacional de 2006) make this species very distinctive within Promoción Científica y Tecnológica. Financial this tribe. support for Dr. Alarie was provided by the Natural Inclusion of the first-instar character states of Sciences and Engineering Research Council of H. planatus into data matrices used in larval studies Canada in the form of a Discovery research grant. of phylogenetic relationships of the Colymbetini We most sincerely thank the anonymous reviewers ( Michat 2005; Alarie and Hughes 2006) reinforces for their helpful comments. the hypothesis of a monophyletic origin for members of this tribe. Larvae of the Colymbetini share a total of 10 non-ambiguous character states with REFERENCES CITED regard to the chosen out-group. These are: (i) pres- Alarie, Y. 1995. Primary setae and pores on the legs, the ence of an occipital suture (character 00, Fig. 2 View Figs ); last abdominal segment, and the urogomphi of (ii) presence of additional ventroapical pores larvae of Nearctic Colymbetinae ( Coleoptera : on antennomere 3 (character 07, Fig. 6 View Figs ); (iii) a Adephaga: Dytiscidae ) with an analysis of their non-protruding lateroventral process (A3′) on phylogenetic relationship. The Canadian Entoantennomere 3 (character 08, Fig. 6 View Figs ); (iv) medial mologist 127: 913–943.
Alarie, Y. 1998. Phylogenetic relationships of Nearctic position of ANg (character 09, Fig. 4 View Figs ); (v) absence
Colymbetinae ( Coleoptera : Adephaga: Dytiscidae ) of MXa (character 11; Fig. 8 View Figs ); (vi) distal articula- based on chaetotaxic and porotaxic analysis tion of the procoxal CO7 (character 15); (vii) proxi- of head capsule and appendages of larvae. The mal origin of FE1 (character 18, Fig. 10 View Figs ); (viii) Canadian Entomologist 130: 803–824. elongate and setiform aspect of the metafemoral Alarie, Y., and S. Hughes. 2006. Re-descriptions of FE6 (character 23; Fig. 11 View Figs ); (ix) presence of larvae of Hoperius and Meladema and phylobasoventral spinulae on trasal claws (character 33, genetic implications for the tribe Colymbetini Figs 10–11 View Figs ); and (x) one-segmented urogomphi ( Coleoptera Dytiscidae ). Memorie della Società (character 37, Fig. 15 View Figs ). Entomologica Italiana 85: 307–334.
Alarie, Y., M. C. Michat,A.N. Nilsson,M. Archangelsky, The phylogenetic relationship of Hoperius to
and L. Hendrich. 2009. Larval morphology of other Colymbetini genera was deemed equivocal Rhantus Dejean, 1833 ( Coleoptera : Dytiscidae : by Alarie and Hughes (2006); however, its posi- Colymbetinae): descriptions of 22 species and tion as a sister taxon to a clade comprised of phylogenetic considerations. Zootaxa 2317: 1–102. Neoscutopterus , Meladema , and Bunites appears Allen, R. T. 1990. Insect endemism in the interior highwell supported, based on this analysis. Indeed, lands of North America. Florida Entomologist both Jackknife (73) and Bremer (3) values indi- 73: 539–569. cate strong support for the monophyletic origin Anonymous. 2013. Average monthly temperatures for of these four genera ( Fig. 16 View Figs ). First instars of this Milledgeville, Georgia, 1981–2010. National
Oceanic and Atmospheric Administration. Available clade have a large number of additional primary
from: nowdata.rcc-acis.org/FFC/pubACIS_results setae on the urogomphus (character 38, Fig. 15 View Figs ), (Accessed 26 September 2013). a unique character state within the Colymbetini . It Barman, E. H. 1996. Life history analysis of Dytiscidae is also noteworthy that first instars of Hoperius in selected habitats. Proceedings of the Fourth share with those of Neoscutopterus , Meladema, International Conference on Classification, Phy- and Bunites four homoplastic character states: (i) logeny, and Natural History of Hydradephaga presence of additional setae on the mandible (char- ( Coleoptera ). Entomologica Basiliensia 19: 31–42. acter 10, Fig.7 View Figs ); (ii) presence of additional dorsal Barman, E. H., B. R. Lemieux, and B. P. White. femoral setae (character 19, Fig. 10 View Figs ); (iii) pres- 2006. Corrections for identification of mature
larvae of Rhantus calidus (Fabricius) and Hoperius ence of short, spiniform additional setae on the
planatus Fall ( Coleoptera : Dytiscidae ) in Georgia. abdominal segment 8 (character 35, Fig. 14 View Figs ); and Georgia Journal of Science 64: 131–134. (iv) absence of ventral rows of spinulae on the Barman, E. H., G. A. Nichols, and R. L. Sizer. meso- and metatibia (character 32, Figs. 10, 11 View Figs ). 1996. Biology, mature larva, and pupa of Agabus
punctatus Melsheimer ( Coleoptera : Dytiscidae ).
Georgia Journal of Science 54: 183–193.
ACKNOWLEDGMENTS De Marzo, L. 1974. Studi sulle larve dei coleotteri Aquatic Coleoptera Laboratory Contribution ditiscidi. II. Morfologia dei tre stadi larvali di No. 79. This project was supported in part by 10 Melanodytes : 57–80. pustulatus Rossi. Entomologica, Bari resources provided by the Department of Bio-
Feduccia, A. 1993. Evidence from claw geometry indilogical and Environmental Sciences, Georgia cating arboreal habits of Archaeopteryx. Science College & State University. Financial support 259: 790–793. for Dr. Michat was provided by project PIP Folkerts, G. W., and L. A. Donavan. 1974. Notes 112-200801-02759 from Consejo Nacional de on the ranges and habitats of some little-known
aquatic beetles of the southeastern U. S. (Coleop- Adephaga). Version 1.I.2013. Umeå: distributed tera: Gyrinidae, Dytiscidae ). The Coleopterists electronically as a PDF file by the author .
Bulletin 28: 203–208. Nilsson, A. N., and W. L. Hilsenhoff. 1991. Review of
Goloboff, P., J. Farris, and K. Nixon. 2008. TNT, a the first-instar larvae of Colymbetini (Coleoptera: free program for phylogenetic analysis. Cladistics Dytiscidae), with a key to genera and phylogenetic 24: 774–786. analysis. Entomologica Scandinavica 22: 35–44.
Jasper, S. K., and R. C. Vogtsberger. 1996. First Plummer, G. L. 1983. Georgia Rainfall: Precipitation Texas records of five genera of aquatic beetles Patterns at 23 places, 1734–1982. The Georgia (Coleoptera: Noteridae, Dytiscidae, Hydrophilidae) Academy of Science and the Institute of Natural with habitat notes. Entomological News 107: Resources and Cooperative Extension Service, 49–60. University of Georgia, Athens, GA.
Kitching, I. J., P. L. Forey, C. J. Humphries, and Spangler, P. J. 1973. The bionomics, immature stages,
D. M. Williams. 1998. Cladistics. Second Edi- and distribution of the rare predaceous water beetle, tion. The Theory and Practice of Parsimony Analy- Hoperius planatus (Coleoptera: Dytiscidae). Prosis. Systematic Association Publications 11. Oxford ceedings of the Biological Society of Washington University Press, New York, NY. 56: 423–434.
Michat, M. C. 2005. Larval morphology and phylogenetic Turnbow, R., and C. L. Smith. 1983. An annotated relationships of Bunites distigma (Brullé) (Coleop- checklist of the Hydradephaga (Coleoptera) tera: Dytiscidae: Colymbetinae: Colymbetini). The of Georgia. Journal of Georgia Entomological Coleopterists Bulletin 59: 433–447. Society 18: 429–443.
Miller, K. B. 2001. On the phylogeny of the Dytiscidae Wall, W. P., E. H. Barman, and C. M. Beals. 2006. (Insecta: Coleoptera) with emphasis on the mor- A description and functional interpretation of phology of the female reproductive system. Insect the mandibular geometry of Agabus punctatus Systematics and Evolution 32: 45–92. Melsheimer, 1844, Rhantus calidus (Fabricius,
Nilsson, A. N. 1986. Life cycles and habitats of the 1792) and Acilius mediatus (Say, 1823), (Coleopnorthern European Agabini (Coleoptera: Dytiscidae). tera: Dytiscidae). Aquatic Insects 28: 277–289.
Entomologica Basiliensia 11: 391–417. Wiley, E. O. 1981. Phylogenetics. The Theory and Prac-
Nilsson, A. N. 1988. A review of primary setae and tice of Phylogenetic Systematics. John Wiley & pores on legs of larval Dytiscidae (Coleoptera). Sons, New York, NY.
Canadian Journal of Zoology 66: 2283–2294.
Nilsson A. N. 2013. A World Catalogue of the family (Received 22 July 2013; accepted 24 March 2014. Pub- Dytiscidae, or the Diving Beetles (Coleoptera, lication date 18 June 2014.)
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |