Ilyocypris japonica Okubo, 1990
|
publication ID |
https://doi.org/10.11646/zootaxa.4652.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:B8EA4D0C-07CC-4C8F-9B21-C97B9852AAFF |
|
DOI |
https://doi.org/10.5281/zenodo.5610190 |
|
persistent identifier |
https://treatment.plazi.org/id/A90187A1-BA46-FF9A-128B-D0804FF6F9FF |
|
treatment provided by |
Plazi |
|
scientific name |
Ilyocypris japonica Okubo, 1990 |
| status |
|
Ilyocypris japonica Okubo, 1990
( Figs 1–4 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 A–I, 5A & B, 6A, 7 & 17)
1989 Ilyocypris sp. A.—Okubo & Ida: 105, pl. 4g–l, fide Okubo 1990.
1990 Ilyocypris japonica sp. nov. —Okubo: 40, fig. 1f–i.
1992 Ilyocypris haterumensis sp. nov. —Okubo & Terauchi: 101, fig. 1a–f, fide Okubo 2004.
? 2002 Ilyocypris dentifera Sars, 1903 —Nakao & Tsukagoshi: 71, figs 2a–d, tables 2–5, herein.
2003 Ilyocypris japonica Okubo, 1990 — Yamaguchi & Endo: Fig. 4g View FIGURE 4 , table 1.
2004 Ilyocypris japonica Okubo, 1990 — Okubo: 17–18, figs 4w–aa.
2007 Ilyocypris japonica Okubo, 1990 —Yamada: 46, figs 2i & 3n, tables 1 & 2.
2008 Ilyocypris japonica —Tinn & Oakley : Fig. 1 View FIGURE 1 .
? 2008 Ilyocypris dentifera Sars, 1903 —Nakao & Tsukagoshi: 251, 263, plate 1a–d, tables 1, 12–14, herein.
2009 Ilyocypris japonica Okubo, 1990 — Hayashi et al.: 77.
2010 Ilyocypris japonica —Kaji & Tsukagoshi : Table 1 View TABLE 1 .
2011 Ilyocypris haterumensis Okubo, 1992 —Martens & Savatenalinton: Table 2.
2011 Ilyocypris japonica Okubo, 1990 —Martens & Savatenalinton: Table 2.
2012 I. haterumensis Okubo 1992 —Karanovic: 204, 207.
2012 Ilyocypris japonica Okubo 1990 a—Karanovic: 205, 208.
2012 Ilyocypris japonica Okubo, 1990 — Sidorov & Semenchenko: 233.
2012 Ilyocypris japonica Okubo, 1990 — Hayashi et al.: 61.
2013 I. haterumensis Okubo, 1992 —Karanovic & Lee: 41, 58, 64.
2013 I. japonica Okubo, 1990 —Karanovic & Lee: 41, 58, 64, appendix.
2013 I. haterumensis—Zhai & Xiao: 468.
2013 Ilyocypris japonica Okubo, 1990 —Martens et al.: No page numbers.
2013 Ilyocypris haterumensis Okubo, 1992 —Martens et al.: No page numbers.
2015 I. haterumensis Okubo, 1992 —D’Ambrosio et al.: 65.
2016b Ilyocypris japonica— Smith et al.: Fig. 2i View FIGURE 2 .
2016a Ilyocypris japonica Okubo, 1990 —Smith et al.: 484–488, 492–495, fig. 6, tables 1 & 2.
2017 I. japonica—Ghaouaci et al.: 145.
2017 I. haterumensis—Ghaouaci et al.: 145.
2018 Ilyocypris japonica Okubo, 1990 —Smith et al.: Appendix.
2019 Ilyocypris japonica Okubo, 1990 —Meisch et al.: 71.
Diagnosis. Carapace anterior margin slightly more inflated than posterior margin, dorsal margin sloping down posteriorly. Surface covered in many well-developed small pits, and a small number of tiny spines near posterior and anterior margins (sometimes missing). Up to six tubercles sometimes present, rounded to angular. Anterior inner calcified lamellae of both valves with lists. Swimming setae of antenna very long, extending significantly beyond end of claws. Sixth limb with five segments (second endopodal segment divided). Seventh limb with one seta on basis, two setae on third segment (= second endopodal segment), and small, hook-like setules distally on same segment. Anterior end of Zenker organ large and inflated, much larger than posterior end. Inner lobe of hemipenis long and thin, with inflated, rounded tip, and noticeably longer than outer lobe, middle and outer lobes overlapping, copulatory process slender, with curved outer edge and sharply pointed tip.
Type locality. A type locality was not explicitly designated by Okubo (1990). The type material was collected from “paddy fields near Tsukuda Station of the Japan Railway, Gumma Prefecture” ( Okubo 1990), approximate coordinates (herein given) 36.5610º N, 139.0427º E.
Type material (as designated by Okubo 1990). Holotype: male (FO-652). Allotype: female (FO-654). Paratypes: male (FO-651), male (FO-655). Numbers were provisional. Location of type material not stated and currently unknown.
Material examined. See Table 1 View TABLE 1 .
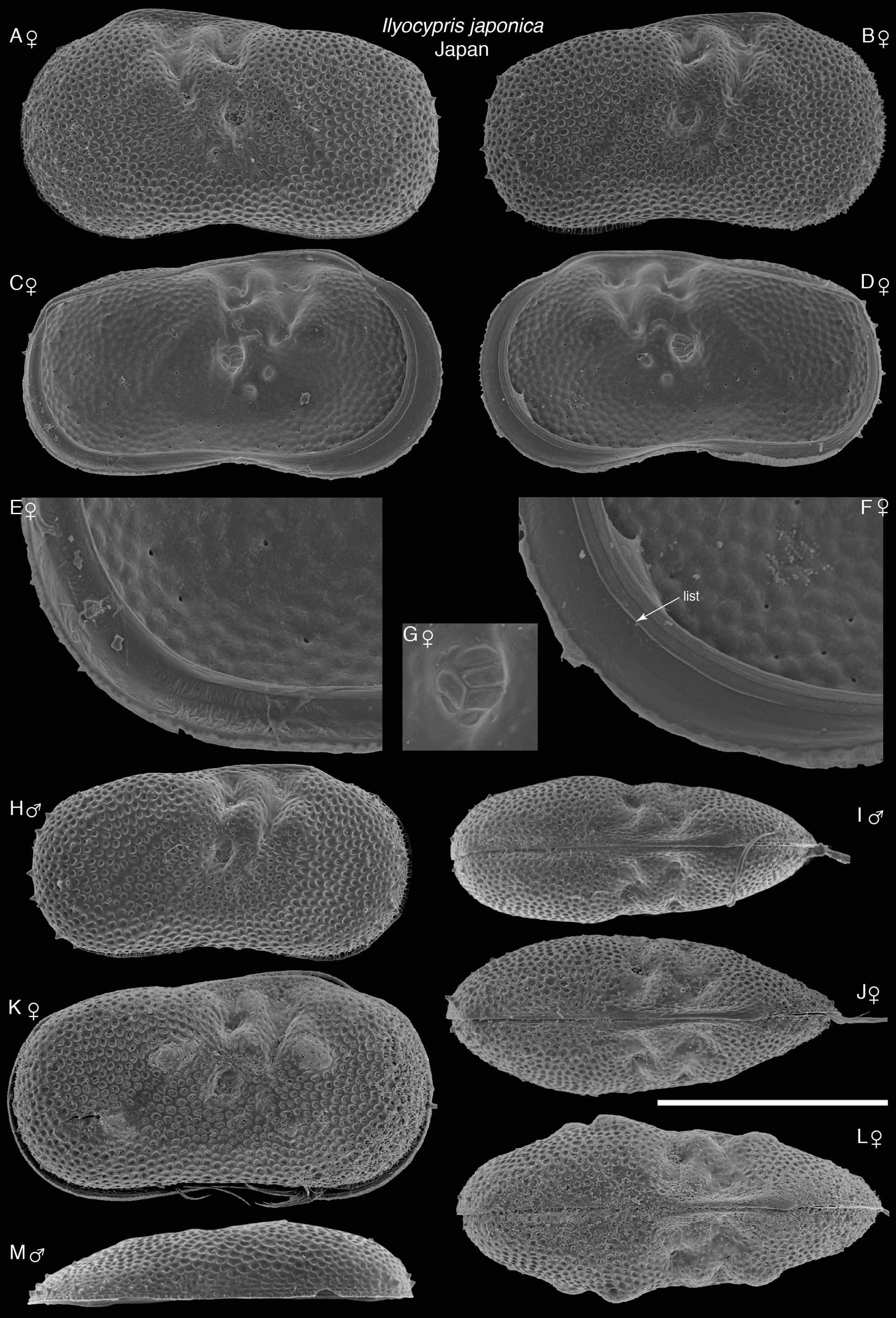
Description. Male carapace length 659–823 µm, height 367–431 µm, height/length 0.50–0.56. Female carapace length 722–927 µm, height 405–500 µm, height/length 0.54–0.56. Anterior margin approximately evenly curved, more inflated than posterior margin ( Figs 1 View FIGURE 1 & 2 View FIGURE 2 ). Dorsal margin noticeably sloping down posteriorly, ventral margin distinctly concave. Maximum height at anterior end of hinge. Well-defined bifurcated sulcus near dorsal margin above and slightly anterior of adductor muscle scars. Both valves without outer lists ( Fig. 1M View FIGURE 1 ). With or without tubercles; when present, one rounded to angular tubercle each side of sulcus (posterior one called lateral tubercle by some authors), one in mid-position of sulcus, one towards postero-ventral region, and two tubercles in central ventral region ( Fig. 2J & K View FIGURE 2 ). Six adductor muscle scars, location visible externally as pit below posterior part of sulcus. Surface of carapace covered with well defined pits, and small spines near anterior and posterior margins. Anterior inner calcified lamellae of both valves with lists. Inner side of lists uneven and with striae. Postero-ventral region of calcified inner lamella of left valve with shallow depression, internally roughened, but not with well-defined marginal ripplets. Dorsal view more rounded posteriorly than anteriorly, maximum width posterior of midlength. Males generally smaller and slightly more elongate than females. Dorsal view of male similar to female, but with more rounded posterior margin and slightly more compressed.
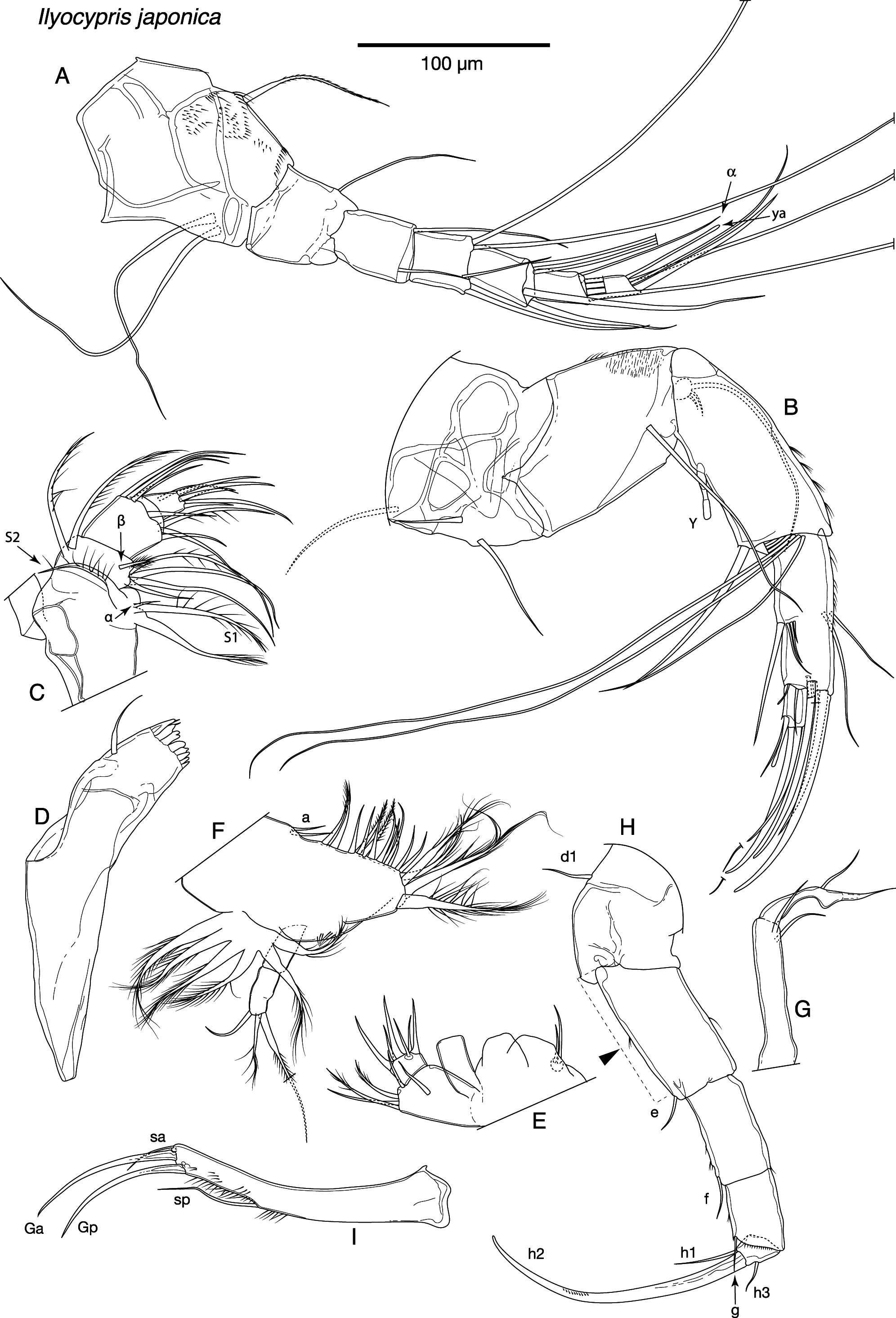
Antennule ( Fig. 3A View FIGURE 3 ) with seven articulated segments. First segment with two long sub-apical setae on ventral margin and one medium-length seta on dorsal margin. Second segment with relatively long apical-dorsal seta. Third segment with one short apical-dorsal seta and one short apical-ventral seta. Fourth segment with two long apicaldorsal setae, and two shorter apical-ventral setae. Fifth segment with two long apical-dorsal setae, and two shorter, stout, apical-ventral setae. Sixth segment with four long setae and relatively long alpha seta. Terminal segment apically with two short setae, one of which quite stout, one long seta and relatively short aesthetasc ya.
Male antennae with fourth segment undivided ( Figs 3B View FIGURE 3 & 4B View FIGURE 4 ). Swimming setae very long, extending considerably beyond end of claws; shorter accompanying seta also long, reaching to mid-point of claws. G3 reduced to short seta, but extending slightly beyond end of terminal segment. Claw z1 small and slender, not reaching to end of claw G2. Female antenna claw G2 long, longer than G1 ( Fig. 4A View FIGURE 4 ). Claw Gm ca. 80% length of claw GM.
Mandibular coxa small, with well-developed teeth on endite ( Fig. 3D View FIGURE 3 ). Mandibular palp four-segmented ( Fig. 3C View FIGURE 3 ). S1 seta on first segment longer than S2, alpha seta small and slender. Second segment with two setae on outer edge, and 3+1+beta setae on inner edge. Beta seta short, stubby and hirsute. Third segment with four setae on outer apical corner, three setae on inner apical corner, and one apical seta between other two groups. Terminal segment with three stout apical setae, two thinner and shorter apical setae and one seta protruding from area proximal of segment mid-length, towards outer edge.
Maxillula palp first segment with three sub-equal, medium-length setae on outer apical corner, one shorter seta positioned on apical edge offset from apical corner, and one sub-apical seta in mid-width position ( Fig. 3E View FIGURE 3 ). Second segment wider than long, apically with three claw-like setae and three shorter setae.
Fifth limb with two a setae on dorsal margin, and approximately 17 setae along dorsal and anterior edge of basis ( Fig. 3F View FIGURE 3 ). Branchial plate with six rays. Palp (endopodite) of female two-segmented, terminating with three setae of differing lengths. Palps of male symmetrical, with slender elongate hooks ( Fig. 3G View FIGURE 3 ).
Sixth limb five-segmented ( Fig. 3H View FIGURE 3 ). First segment (= protopodite) with short d1 seta. Second, third and fourth segments (= first, second and third endopodal segments respectively) with short e, f and g setae respectively. Terminal segment (= fourth endopodal segment) with relatively long h1 seta, short h3 and well-developed h2 claw, 1.9x length of anterior sclerotized margin of first endopodal segment (indicated with black triangle on Fig. 3H View FIGURE 3 ).
Seventh limb with four segments ( Fig. 4C View FIGURE 4 ). One seta (d1) on basal segment. Second segment with one apical seta (e). Third segment with two setae on outer edge (f and g) (junior synonym I. haterumensis figured with one), and group of hook-like setules apically. Fourth segment with two long and one medium-length setae apically.
Caudal ramus typical of genus, ramus curved ( Fig. 3I View FIGURE 3 ). Claw Gp approximately same length as Ga, seta sp long.
Female reproductive organ with one flattened lobe, sub-angular towards anterior and posterior corners ( Fig. 4D View FIGURE 4 ). Organ delicate and easily distorted or damaged during dissections.
Hemipenes with outer lobe rounded distally, overlapping middle lobe ( Figs 4 View FIGURE 4 E–I, black triangle indicates overlap, 5A & B). Middle lobe sub-quadrate to triangular, with thick base, widening distally, distal inner corner sharply angled, outer edge angled to rounded. Inner lobe long and thin, protruding beyond outer lobe, terminating with bul- bous end; distally variable, ranging from narrow and hook-like to slightly thicker and bulbous. Copulatory process slender, with curved outer edge and sharply pointed tip.
Zenker organ with proximal end significantly inflated compared with posterior end, and with 15–17 internal rosettes ( Fig. 6A View FIGURE 6 ). Spermatozoa lengths ranging from 1007 to 1232 µm with an average of 1139 µm ( Smith et al. 2016a).
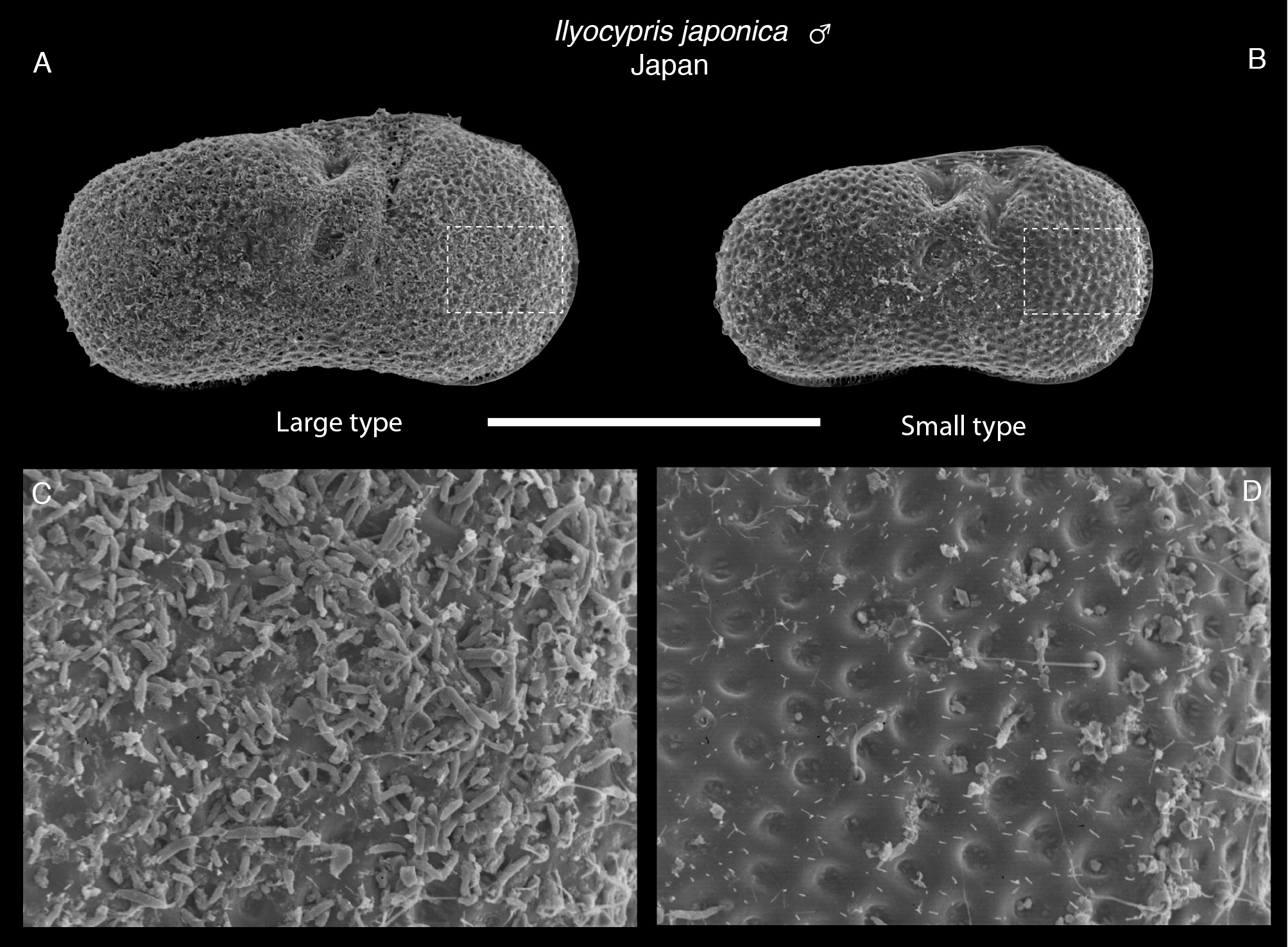
Intraspecific variation. 13% of specimens studied have tubercles present on each valve (see description for more details) ( Table 1 View TABLE 1 ), ranging from slightly to well developed, rounded to angular ( Figs 1 View FIGURE 1 & 2 View FIGURE 2 ). The inner and middle lobes of the hemipenis are somewhat variable in shape; some specimens have a middle lobe that is slightly wider distally than others, and the outer corner ranges from quite angular to rounded ( Fig. 4E, G & I View FIGURE 4 ). The male copulatory process is slightly narrower distally in some specimens compared with others. Sizes of individuals were variable, even within one sample, with some adult specimens noticeably larger (approximately 20% longer and 14% higher) than other adults ( Fig. 7 View FIGURE 7 ). The hemipenis morphology of the two size types are very similar ( Fig. 5A & B View FIGURE 5 ), so we conclude that they are the same species (see Discussion). The roughened surface of the postero-ventral region of the calcified inner lamella of the left valve varies in extent, extending higher up the posterior margin in some specimens.
Remarks. The locations of the type specimens of Ilyocypris japonica and its junior synonym Ilyocypris haterumensis Okubo, 1992 (in Okubo & Terauchi 1992) were not mentioned in the original publications and enquires were unsuccessful in determining their whereabouts. The material must therefore be considered lost, and only the figures in Okubo (1990; 2004) and Okubo & Terauchi (1992) are available for identification of this species.
In the original description of I. haterumensis it was noted that it was similar to I. japonica , but the middle lobe of the hemipenis was slightly different, and this was used to justify the erection of a new species ( Okubo & Terauchi 1992). However, it was later synonymised with I. japonica by Okubo (2004), but without an explanation. The middle lobe of the hemipenis of I. japonica is somewhat variable in shape ( Figs 4E, G & I View FIGURE 4 ) and the figured middle lobe of I. haterumensis does fit into this range. In the figures of I. haterumensis provided by Okubo & Terauchi (1992) the seventh limb has one seta on the third segment, and the sp seta on the caudal ramus is short, while I. japonica has two setae on the same seventh limb segment, and the sp seta on the caudal ramus is noticeably longer. These discrepancies cannot be checked because of the lack of type material. We therefore follow Okubo (2004), who observed specimens of both species and considered that the two names are synonyms. We assume that the discrepancies are due to errors, damage or abnormalities with the specimen of I. haterumensis figured by Okubo & Terauchi (1992). This is supported by our material, some of which has a very similar hemipenis morphology to that of I. haterumensis (i.e. a very similar middle lobe morphology), but two setae on the third segment of the seventh limb and a long sp seta on the caudal ramus.
Ilyocypris japonica is morphologically very similar to Ilyocypris mongolica Martens, 1991 . Ilyocypris mongolica differs from I. japonica in the following characters: it is slightly more rectangular in lateral view, with a shallower sloping dorsal margin; the pitting on the carapace is coarser; the inner lists on the calcified inner lamella are closer to the inner edge; and the copulatory process, although similar, is differently shaped ( Figs 4J & K View FIGURE 4 , 5C View FIGURE 5 , 8 View FIGURE 8 ). The middle lobe of the hemipenis is also generally larger distally than that of I. japonica , but the variation of this character in I. japonica is quite large, so that the largest example is similar to that of I. mongolica . These morphological differences are small, but due to the different geographical (and climatic) distributions and habitats of the two species (see Distribution section below), we provisionally treat them as two separate species.
Contrary to our results, a cladistic study by Karanovic & Lee (2013) of 21 Ilyocypris species indicated that I. japonica and I. mongolica are only distantly related (see cladistic diagram Fig. 15 View FIGURE 15 of Karanovic & Lee 2013). However, characters for some species were incorrectly coded in the study of Karanovic & Lee (2013) (e.g. for I. japonica characters 3, 11, 12, and 14 were incorrectly coded), and this undoubtedly accounts for the discrepancy between their cladistic results and our detailed morphological analysis. A corrected cladistic analysis of Ilyocypris species is not given herein, as an informative analysis is hindered by the lack of comprehensive descriptions for many species.
The record of I. dentifera reported from the brackish part of the Obitsu River estuary in Japan by Nakao & Tsukagoshi (2002; 2008) is possibly a misidentification of I. japonica . See under I. dentifera below for more details.
Distribution. In Japan I. japonica is known from Gunma, Okinawa and Shiga Prefectures ( Okubo & Ida 1989; Okubo 1990; Okubo & Terauchi 1992; herein) ( Fig. 9 View FIGURE 9 ). The records of I. japonica reported herein from Gyeongsangnam-do and Gyeongsangbuk-do in the south east of South Korea are the first for that country (see Table 1 View TABLE 1 for more details). Its distribution corresponds to temperate to cold Köppen climatic zones with hot summers, and no dry season (zones Dfa and Cfa; Peel et al. 2007). The morphologically similar species I. mongolica , in contrast, is known from Mongolia and Inner Mongolia, China ( Martens 1991; Van der Meeren et al. 2010; Zhai & Zhao 2014), corresponding to Köppen climatic zones of arid, cold steppe (BSk), arid, cold desert (BWk) and cold, dry winters, with warm summers (Dwb) ( Peel et al. 2007) ( Fig. 9 View FIGURE 9 ).
Ecology. This species can be abundant in rice fields in Japan, found in the top few millimetres of mud in the oxygenated zone. In Japan it has only been found in rice fields, but in Korea it was also collected from a lotus field ( Table 1 View TABLE 1 ). This species has so far only been found from mid May through to late July ( Okubo 1990; herein), although its appearance and disappearance in rice fields is probably strongly influenced by the rice-growing cycle rather than the seasons per se. Ilyocypris mongolica , on the other hand, has not been reported from rice fields, but from a puddle, lakes, and a saline ditch on the flood plain of a river ( Martens 1991; Van der Meeren et al. 2010; Zhai & Zhao 2014).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Cypridocopina |
|
Family |
|
|
SubFamily |
Ilyocypridinae |
|
Genus |
Ilyocypris japonica Okubo, 1990
| Smith, Robin James, Zhai, Dayou & Chang, Cheon Young 2019 |
Ilyocypris haterumensis
| Okubo 1992 |
I.
| Okubo 1992 |
I.
| Okubo 1992 |
Ilyocypris haterumensis
| Okubo 1992 |
I.
| Okubo 1992 |
Ilyocypris mongolica
| Martens 1991 |
Ilyocypris mongolica
| Martens 1991 |
I. mongolica
| Martens 1991 |
I. mongolica
| Martens 1991 |
Ilyocypris japonica
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
I.
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
Ilyocypris japonica
| Okubo 1990 |
I. japonica
| Okubo 1990 |
I. japonica
| Okubo 1990 |
I. japonica
| Okubo 1990 |
I. japonica
| Okubo 1990 |
I. japonica
| Okubo 1990 |
Ilyocypris
| Brady & Norman 1889 |