Scopula ascensionis, Karisch & Kramp, 2019
|
publication ID |
https://doi.org/ 10.21248/contrib.entomol.69.1.087-090 |
|
DOI |
https://doi.org/10.5281/zenodo.5742803 |
|
persistent identifier |
https://treatment.plazi.org/id/AA2487BF-FFC0-BB5C-358E-F8F5FD23DCCE |
|
treatment provided by |
Felipe |
|
scientific name |
Scopula ascensionis |
| status |
sp. nov. |
Scopula ascensionis spec. nov.
urn:lsid:zoobank.org:pub:6CA2D311-B23A-4000-9A0A-B0D04EAC27C8
Holotype: „ ASCENSION IS. | E.A.G. DUFFEY, | B.M. 1958-760.“, „ NHMUK 010919255 About NHMUK “, „Gen.-Präp. 3582 | präp. KARISCH, 2018 “. In coll. NHMUK.
Paratypes: Ascension Island, Devil’s Ashpit near NASA building, 1 (gen. prep. 3616, KARISCH) 2017-02-20, at night, leg. T. KARISCH (MNVD); same locality, 1 2017-05-31, e. o., leg. T. KARISCH (coll. KARISCH, Demitz-Thumitz) .
Description (Figs 3, 4, 5): Frons dark brown. Palps dark brown, as long as the diameter of the eye. Vertex and collar pale ochre.
Antenna of pale ochre, ventral with cilia, about twice width of flagellum. Ventral side of first segment of thorax dark brown.
Wingspan 18–19 mm. Forewing narrow and elongated. Wings sand coloured, forewing slightly more brown towards costa; no distinct lines, but postmedian indicated as a light shade; minute black discoidal spot on fore and hindwing. Fringes sand coloured.
Underside of the forewing sand coloured, more or less greyish dusted.
Hind tibia of 2nd pair of legs with two strong spurs at the end; 3rd pair densely covered with white scales, no spurs.
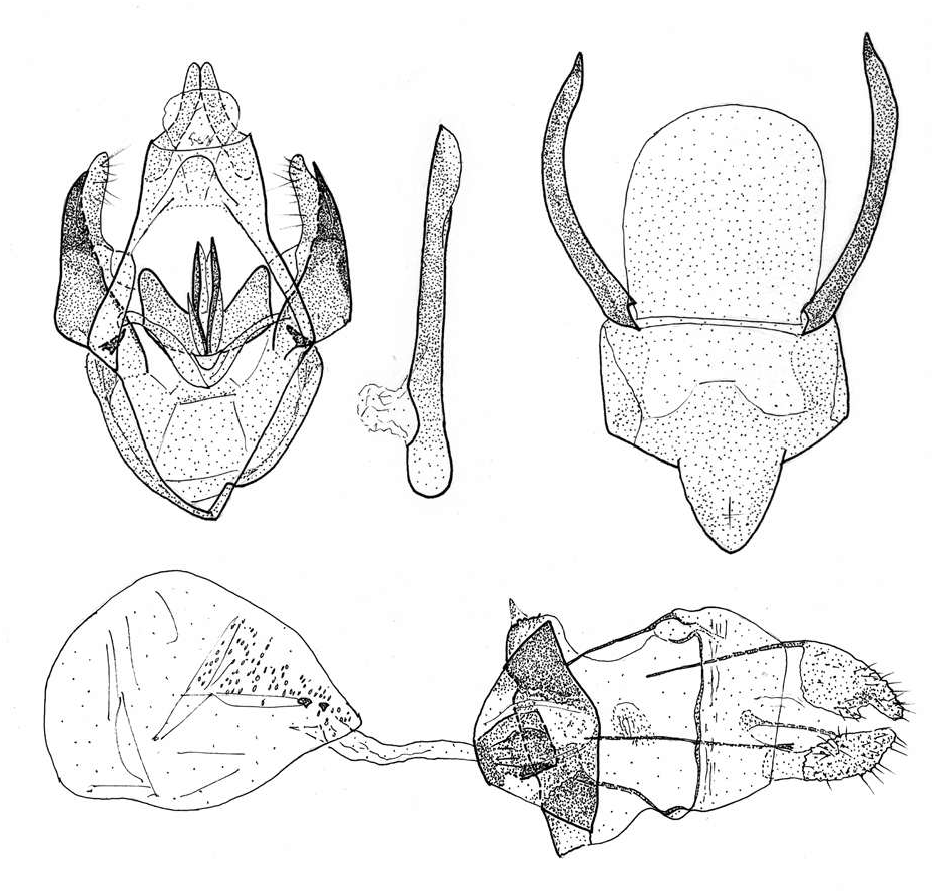
Male genitalia ( Fig. 6 View Fig ): Socii of medium length. Fibula narrow, strong, slightly shorter than valvula. Aedeagus slender, without cornuti. Sternum A8 with nearly uniform ceras, longer then mappa.
Female genitalia (Fig. 7): Lamella antevaginalis pyramidal. Antrum comparatively broad, well sclerotized. Corpus bursae oval, with very small scale-like sclerites and two stronger teeth.
Similar species: Compared with available sequences in BOLD, the closest matches were with barcodes from Scopula minorata (BOISDUVAL, 1833) (similarity: 98.9 %; sample ID not public) and S. serena PROUT, 1920 (similarity: 98.6 %; sample ID: BC ZSM Lep 63796).
In comparison with S. minorata , Scopula ascensionis spec. nov. does not have the transverse lines on the wings and the black terminal dots. In male genitalia, S. ascensionis spec. nov. can be distinguished from S. minorata by the broader fibula and the uniform ceras, but this might be variable in S. ascensionis spec. nov. too. In female genitalia, S. ascensionis spec. nov. is very different in the pyramidal lamella antevaginalis, the broader antrum, the lack of spinules and the strongly sclerotized structure towards the ductus bursae.
S. ascensionis spec. nov. differs from S. serena in the absence of brown transverse lines on the wings, the discoidal spots are much smaller and the wings are more narrow than in S. serena . The male genitalia of S. ascensionis spec. nov. are very similar to those of S. serena , but the tegumen is longer and the fibula is more stout. Because these characters are consistent in all three known specimens of S. ascensionis spec. nov. we consider that our data supports classification on species-level, and not as a subspecies of S. serena .
6
7 S. ascensionis spec. nov. differs from S. lactaria (WALKER, 1861) in the absence of transverse lines and black terminal dots. The aedaeagus of S. lactaria is smaller, the basal part of the fibula elongated and the ceras shorter.
S. separata (WALKER, 1875) an endemic Scopula from St. Helena, is easy to distinguish from S. ascensionis spec. nov. by the distinct pattern of the wings and the very different male genitalia (female not studied).
Remarks: As mentioned above, a female was caught at the headlights of a car at Devil’s Ashpit in 2017. It was placed in a small tube and laid a few eggs. Three larvae hatched, but breeding them was very difficult, because the collector had to leave the island for St. Helena. On St. Helena, the larvae were provided with a variety of different plants. They accepted Atriplex semibaccata and this plant was used as a food plant during the three weeks stay on the island (on Ascension Island two species of Chenopodioideae occur: Dysphania ambrosioides (L.) MOSYAKIN & CLEMANTS and Chenopodium murale L., the first one not too far away from the place where the Scopula was collected – SIM in litt., 2018). After moving to Cape Town, this food plant was no longer available, and an unidentified Atriplex species from a rural locality in the town substituted; as a result, two of the larvae died. The last remaining larva was fed in Germany on another Atriplex and pupated at the beginning of May. The moth (a female) hatched on 31st May, indicating that under artificial conditions the development of the species from egg to imago takes about two months.
The larva ( Figs 8 View Fig , 9) is grey with a dark brown median dorsal line, accompanied by a paler brown line on each side; laterally, more or less greyish brown with grey sinuous lines. The head capsule and anal prolegs are chestnut.
Pupa yellowish brown, with the Scopula -characteristic diverging pair of cremastral setae ( HAUSMANN 2004) (Figs 10, 11).
Etymology: Named after the island, where it occurs.
| NHMUK |
Natural History Museum, London |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |