Pekinomyia syringae Jiao & Kolesik, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4742.1.13 |
|
publication LSID |
lsid:zoobank.org:pub:CF039F48-5BC7-491B-A349-49881D86E519 |
|
DOI |
https://doi.org/10.5281/zenodo.3681165 |
|
persistent identifier |
https://treatment.plazi.org/id/AE971B12-CA82-4C49-A99D-D37EB26792B3 |
|
taxon LSID |
lsid:zoobank.org:act:AE971B12-CA82-4C49-A99D-D37EB26792B3 |
|
treatment provided by |
Plazi |
|
scientific name |
Pekinomyia syringae Jiao & Kolesik |
| status |
sp. nov. |
Pekinomyia syringae Jiao & Kolesik View in CoL , sp. nov.
( Figs 1–19 View FIGURES 1–7 View FIGURES 8–15 View FIGURES 16–19 )
http://zoobank.org/ urn:lsid:zoobank.org:act:AE971B12-CA82-4C49-A99D-D37EB26792B3 (to be added once manuscript is accepted)
Material studied. Holotype male, China, Beijing, Haidian District, Xiangshan, Beijing Botanical Garden (40°00′01″N, 116°12′13″E), collected by Huai-Jun Fu as larva feeding in leaf gall on Syringa reticulata subsp. pekinensis , III-IV.2017, reared in laboratory, deposited in TAUC. GoogleMaps
Paratypes: 9 males, 12 females, 8 larvae, same data as holotype GoogleMaps ; 8 pupae, same data as holotype but collected II.2017 GoogleMaps ; 10 larvae, same data as holotype but collected 8.X.2017 GoogleMaps ; 10 larvae, same data as holotype but collected 15.X.2017 GoogleMaps .
Description.
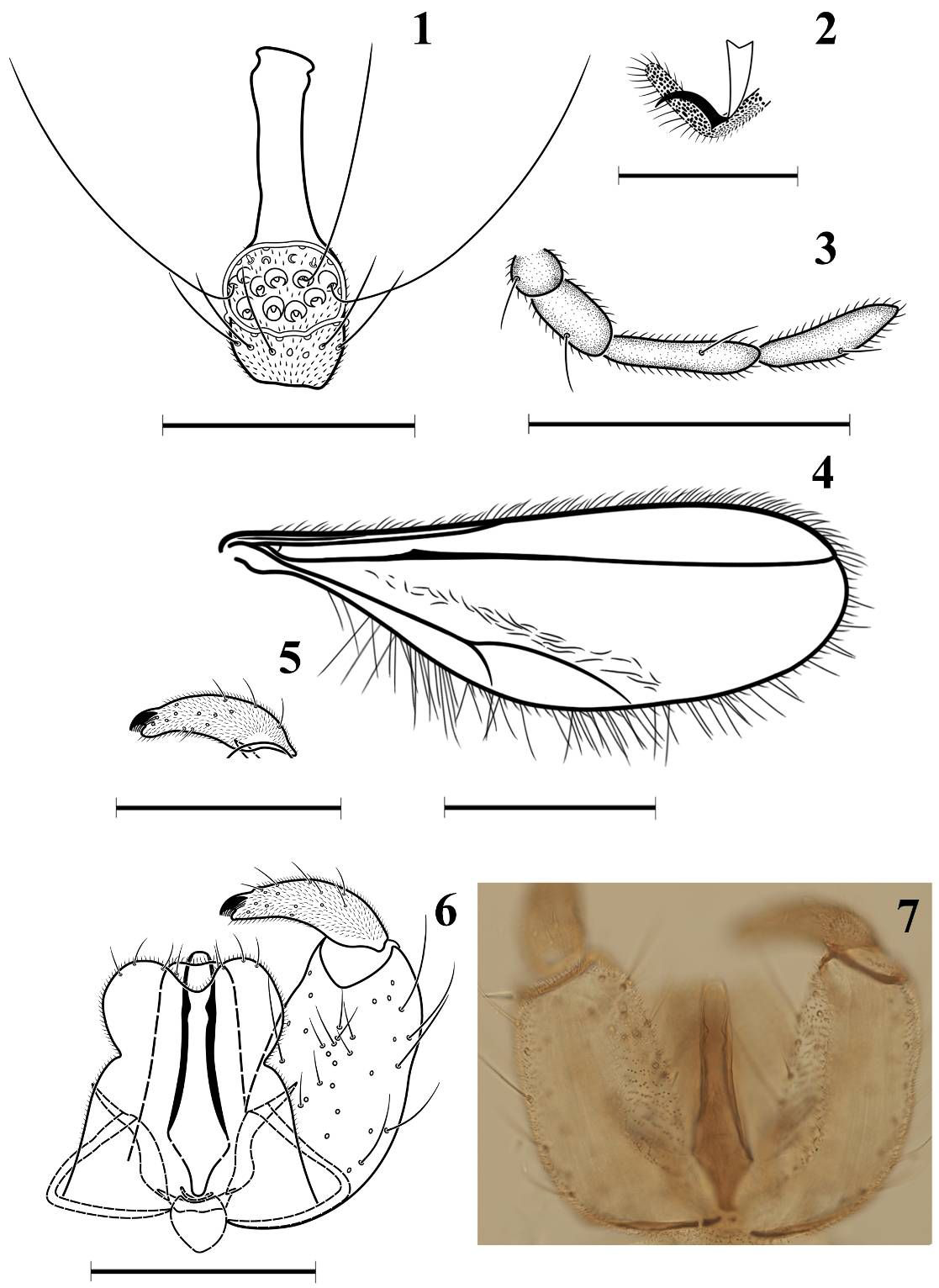
Male ( Figs 1–7 View FIGURES 1–7 , 17 View FIGURES 16–19 ). Colour of abdomen dark orange ( Fig. 17 View FIGURES 16–19 ). Wing length 1.4–1.7 mm (n=10), 2.9–3.2x longer than wide.
Head. Eye bridge 6–7 facets long. First palpal segment shortest, third and fourth longest ( Fig. 3 View FIGURES 1–7 ). Antenna: flagellomeres 14 or 15 in number, circumfila consisting of two horizontal and two vertical, interconnected bands, neck 1.3x length of node ( Fig. 1 View FIGURES 1–7 ).
Thorax. Wing ( Fig. 4 View FIGURES 1–7 ) hyaline, with short setae on anterior edge and long setae on posterior edge, covered with narrow scales, R 1 joining C slightly proximad of wing mid-length, R 5 slightly bent anteriorly near juncture with C, R S in form of angular thickening on R 5 positioned closer to distal end of R 1 than arculus, wing fold recognizable only as area with denser scales, M 4 and CuA forming fork. Legs densely covered with narrow scales and setae. Empodium substantially longer than tarsal claws ( Fig. 2 View FIGURES 1–7 ).
Abdomen. First through sixth tergites rectangular, with single posterior row of long setae, with anterior pair of trichoid sensilla, no lateral and central setae, without scales; seventh tergite as sixth but slightly smaller; eighth considerably smaller than seventh, bare except anterior pair of trichoid sensilla; second through seventh sternites rectangular, with irregular but mostly single posterior row of long setae, dense lateral and several central setae, with anterior pair of closely set trichoid sensilla; seventh sternite as for sixth but slightly smaller; eighth sternite as seventh but considerably smaller. Terminalia ( Figs 5–7 View FIGURES 1–7 ): gonocoxite more than twice as long as wide, without mesobasal lobes; gonostylus slightly tapering posteriorly, arched, fully setulose, sparsely setose, bearing strong comb-like tooth distally; aedeagus about as long as gonocoxite, evenly wide at distal half, rounded apically, strongly sclerotized dorsally and laterally; cerci slightly shorter than aedeagus, round, separated by shallow U-shaped incision, each with several long apical setae; hypoproct as long as cerci, shallowly emarginated, with pair of short setae sub-apically.
Female ( Figs 8–11 View FIGURES 8–15 , 16 View FIGURES 16–19 ). Colour of abdomen red ( Fig. 16 View FIGURES 16–19 ). Wing length 1.4–1.9 mm (n=12), 2.7–2.9x longer than wide.
Head. Eye bridge 6–7 facets long. Flagellomeres 11 or 12 in number, with very short neck; circumfila consisting of two transverse and two or three longitudinal, interconnected bands ( Figs 8, 9 View FIGURES 8–15 ).
Abdomen. Eighth sternite unsclerotized, with anterior pair of closely set trichoid sensilla. Ovipositor robust, slightly shorter than eighth segment, fused cerci round in lateral view ( Fig. 11 View FIGURES 8–15 ) and rounded-square in dorso-ventral view ( Fig. 10 View FIGURES 8–15 ), densely setose; hypoproct rounded-trapezoid in dorso-ventral view, with pair of short apical setae.
Pupa ( Fig. 15 View FIGURES 8–15 ). Head and thorax yellow brown, abdomen whitish yellow at early stage and orange yellow at later stage. Length 1.3–1.5 mm, width 0.5–0.8 mm (n=8). Antennal horns acute. Prothoracic spiracle 4x longer than wide at base, trachea reaching apex. Abdominal spiracles not protuberant.
Mature larva ( Figs 12–14 View FIGURES 8–15 ). Colour yellow. Length 1.1–2.0 mm, width 0.4–0.7 mm (n=8). Antennae tapered, 2.1–2.3x longer than wide at base, head capsule obtusely rounded, posterolateral apodemes 1.1–1.2x longer than head capsule ( Fig. 12 View FIGURES 8–15 ). Sternal spatula ( Fig. 13 View FIGURES 8–15 ) with two rounded anterior teeth divided by shallow U-shaped incision, anteriorly on each side with three setose and two asetose lateral papillae. Terminal segment with two pairs of dorsal setose papillae ( Fig. 14 View FIGURES 8–15 ).
Egg. Spindle-form, translucent, light orange just after deposition, then gradually turning to red, 0.11–0.16 mm in length and 0.060 –0.065 mm in width ( Fig. 16 View FIGURES 16–19 ).
Etymology. The specific name of the new species is derived from the generic name of the host plant.
Remarks. Syringa hosts two other Cecidomyiidae , belonging to different supertribes than the new species ( Gagné & Jaschhof 2017): Asphondylia ligustrinae Kovalev and Mycodiplosis ligustrinaphila Fedotova , both feeding on S. reticulata subsp. amurensis (Rupr.) P.S.Green & M.C.Chang (as Ligustrina amurensis ) in eastern Russia ( Kovalev 1964, Fedotova & Sidorenko 2004).
DNA. The intraspecific similarity of the new species was 98.25–100.00% in the COI sequence ( GenBank accession numbers MN 648492 View Materials – MN 648501 View Materials , sequence length 631 bp) and 98.65-100% in the 12 S sequence (GenBank accession numbers MN 648502 View Materials – MN 648505 View Materials , sequence length 370 bp). Thus, the intraspecific divergence of the type population of Pekinomyia syringae was 0–1.75% in COI and 0–1.35% in 12S.
The new species differed substantially from all sequences available in GenBank and BOLD. In the COI sequence, the new species was closest to an undescribed Cecidomyiinae sp. (GenBank: KJ166670 View Materials ; BOLD: ACB2773 [identified as Asteromyia sp.] voucher BIOUG03738-B10) with the interspecific similarity of 92.74–93.30% (= divergence of 6.70–7.26%. In the 12S sequence, the new species was closest to Lasioptera carophila (GenBank MG684475 View Materials , sequenced by Sikora et al. 2019) and Dasineura trifolii (GenBank MG684469 View Materials , sequenced by Sikora et al. 2019) with the interspecific similarity of 81.51–82.07% and 81.56% (=divergence of 17.93–18.49% and 18.44%, respectively). These low levels of similarity in COI and 12S show that the new species is not closely related genetically to any currently sequenced Cecidomyiidae .
Life history ( Figs 16–19 View FIGURES 16–19 ). Larvae of Pekinomyia syringae induce yellow pustulate galls on the upper side of leaves of Syringa reticulata subsp. pekinensis . The pustules are irregular, 2.0–3.0 mm in diamater and about 0.5 mm high, containing a chamber with a single larva ( Fig. 18 View FIGURES 16–19 ).
In 2017, in the Beijing Botanical Garden, up to 120 galls per leaf were found during an outbreak. Adults emerged from the soil beneath the host plant from late March to mid-April. Females laid scattered eggs on the underside of young leaves ( Fig. 16 View FIGURES 16–19 ). The larval stage lasted from mid-April to October. There appeared to be a single generation per year. In October larvae left galls for the ground where they spun a cocoon, mainly within the top 10 mm of the soil, to overwinter. Pupation took place in the soil and adults emerged from ground’s surface between late February and late March the following year. Currently, the new species is known to occur only in Beijing’s districts of Haidian, Changping, Yanqing, Huairou and Miyun where it has been damaging the street, park and landscape greenery. During the 2017 outbreak, over 90% of trees were affected in the Beijing Botanical Garden. Since then, chemical and physical control measures have been implemented to reduce the infestation.
| MN |
Museu Nacional, Universidade Federal do Rio de Janeiro |
| S |
Department of Botany, Swedish Museum of Natural History |
| COI |
University of Coimbra Botany Department |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |