Martiodendron mediterraneum (Mart. ex Benth.) R.C. Koeppen, 1962
|
publication ID |
https://doi.org/ 10.11646/phytotaxa.578.1.2 |
|
DOI |
https://doi.org/10.5281/zenodo.7542609 |
|
persistent identifier |
https://treatment.plazi.org/id/B43787C8-7C26-FFD9-FF50-703040D3FAA9 |
|
treatment provided by |
Plazi |
|
scientific name |
Martiodendron mediterraneum (Mart. ex Benth.) R.C. Koeppen |
| status |
|
Martiodendron mediterraneum (Mart. ex Benth.) R.C. Koeppen View in CoL View at ENA . Brittonia 14(2): 203 (1962).
≡ Amphymenium mediterraneum Mart. ex Benth. Commentationes de Leguminosarum Generibus View in CoL 31 (1837) (Pre-print of the paper from Ann. Wiener Mus. Naturgesch. 2: 95. 1838).
Type:— Brazil, Bahia: in sylvis Catingas, Serra da Tiuba (Itiuba), III-IV-1819, Martius, C. V. 73 M-0217681 (Lectotype Designated by Koeppen & Iltis [1962: 203]: M!; possible Isolectotypes: M!). ( Figures 8–9 View FIGURE 8 View FIGURE 9 ) View Materials .
= Martiodendron parvifolium (Benth.) Gleason. Phytologia View in CoL 1(3): 141 (1935). ≡ Martiusia parvifolia Benth. View in CoL in Hook. Journal of Botany, being a second series of the Botanical Miscellany 2: 102–103 (1840). ≡ Martia parvifolia Benth. View in CoL in Hook. Journal of Botany, being a second series of the Botanical Miscellany 2: 146 (1840). Type:— Brazil: Piauí: Oeiras. III-1839, Gardner, G. 2149 K000056164 (Lectotype here designated: K!; Isolectotypes: G; HAL!; K!; NY!; P!; US!; W!).
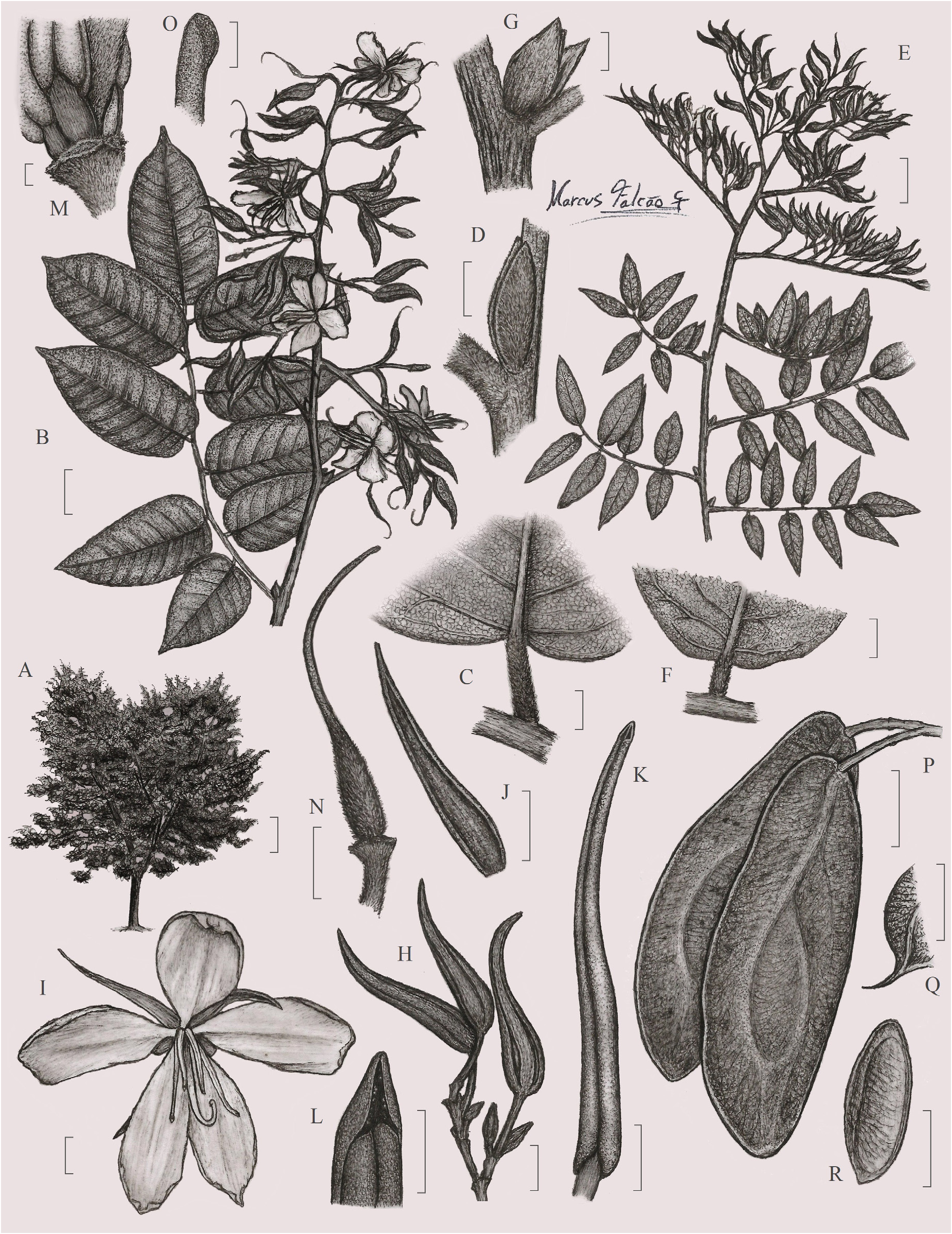
Shrubs to small trees 2–12 m tall, less commonly 12–20(–25) m tall trees, up to 35(–50) cm in diameter, usually without buttresses. Leaf rachis (3.5–)6–12(–18) cm long; petiole (1–)1.5–2(–2.8) cm long; petiolule (1–) 2–5 mm long; leaflets (6–)7–9(–11), the blades chartaceous to coriaceous, the terminal ones (2.5–)5–9.5(–11) × (1.1–)2–3.7(– 5.5) cm, ca. (1.3–)2–3.8 times longer than wide, oblong to elliptical to narrowly oblong to ovate, base obtuse to truncate or cordate, apex acuminate to cuspidate to acute, margin often wavy; leaflets adaxially glabrous, slightly pubescent abaxially; axillary buds elliptic to oblong in outline, 4–5.7(–10) × 1.5–3 mm, apex acuminate to cuspidate. Inflorescences thyrsoid, distichous, terminal, with elongated primary and secondary axis from which cymes form, generally taller than wider, sometimes wider than taller, 7–16(–35) × 6–14(–22) cm. Flower buds (1.3–) 1.9–2.5 cm long, straight, apex commonly curved, less commonly straight, acute to acuminate; sepals 1.3–2.5 × 0.12–0.3 cm; petals 1.7–2.4(–3.5) × 0.5–2 cm; stamens 4, rarely 4+1 abaxial staminode, very rarely 5 stamens, anthers glabrous or very rarely with sparse and inconspicuous indumentum, usually unequal, the two lateral stamens larger and the two adaxial ones smaller, (1–)1.3–1.9 × 0.1–0.2 cm; carpel 5–10 × 1–3 mm, fully pubescent, style 6–15 mm long. Fruits oblong to elliptical to slightly asymmetrical, 7.5–10.5(–14) × (2.7–)3.4–5(–5.7) × 0.3–0.8 cm, 2–2.8(–3.5) times longer than wide, red to vinaceous to purple, wings (0.4–) 0.7–1.4 cm wide in the middle portion of the fruit, dorsal and ventral wings equal or strongly unequal in width, seminiferous nucleus occupying the central part of the fruit, ca. 1.7–2.5 times wider than the widest of the two wings in the middle portion of the fruit, apex obtuse to cuspidate.
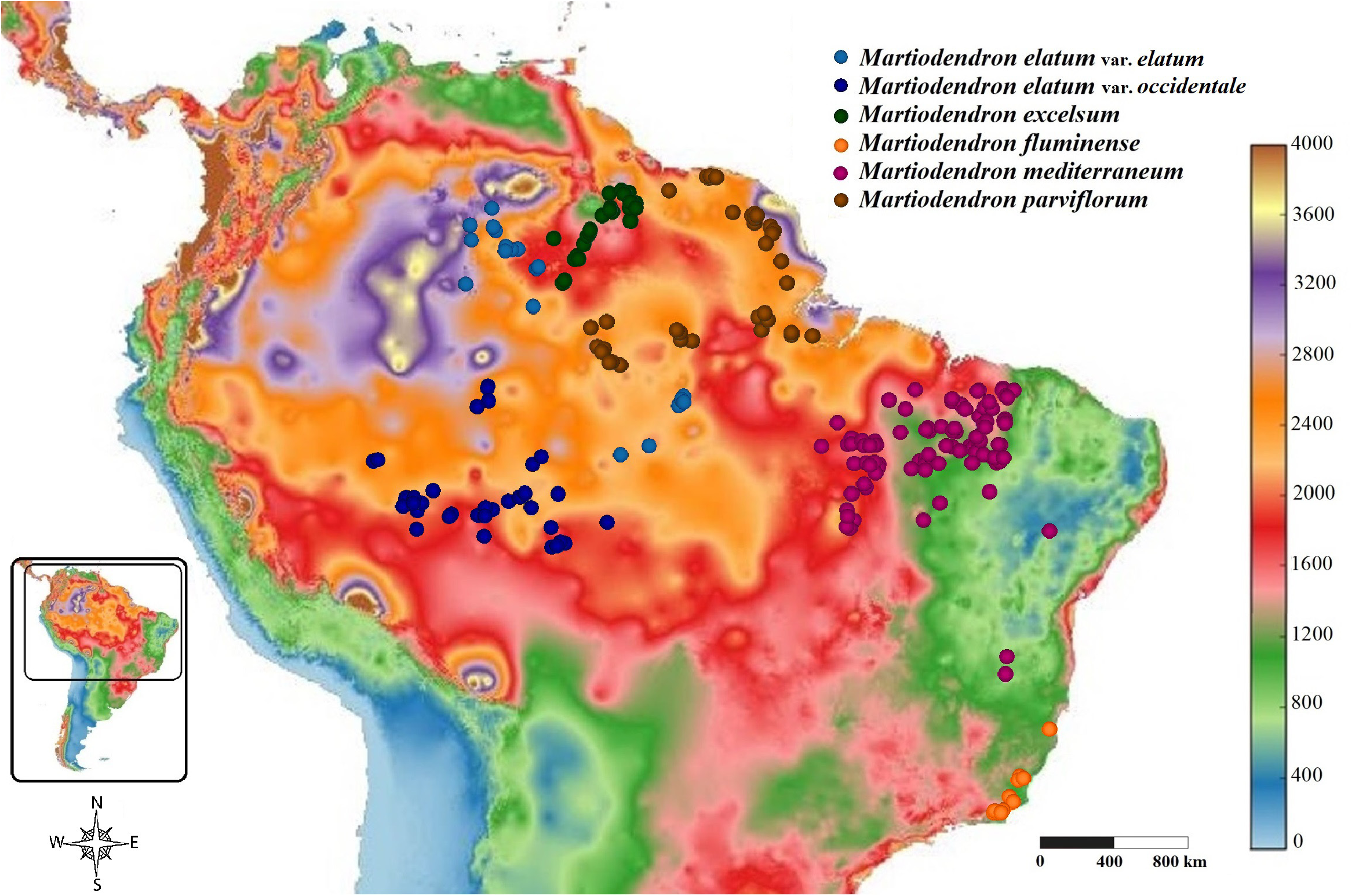
Distribution, Habitat and Ecology: —The species is endemic to Brazil, occurring in the states of Tocantins, Piauí, Maranh ã o, extreme east of Pará, and extreme north of Minas Gerais. The genus was found once in Bahia (Martius 73) but is possibly extinct in the region and has not been collected there for over 150 years ( Lewis, 1987; Queiroz, 2009). However, some areas in northern Bahia are still preserved and poorly collected, including in the Serra da Tiuba/Itiuba, the area where Martius collected the specimens, which may indicate that the species still exists in Bahia ( Figure 15 View FIGURE 15 ). It occurs mainly in the Cerrado but also in transitional areas with the Amazon, Caatinga, and Mata Atlântica. It is generally found in seasonal semideciduous forests and wooded or steppe savannas, sometimes rocky outcrops, in gallery forests or “carrasco” forests in the transition from Cerrado to Caatinga and, very rarely, in arboreal Caatinga stricto sensu, generally found in sandy or rocky soils, less commonly on clayey soils. It is considered frequent or very frequent in some areas of Maranh ã o (Mello 5324; A. Carvalho 631), Piauí (M.S.B. Nacimento 256; L. Santos 491; R. Barros 3313) and Tocantins (H.S. Irwin 21125).
M. mediterraneum is the species of the genus to occur in drier areas in the transition from Cerrado to Caatinga. Still, it is also the one with the greatest range of environments along its distribution, with its continuous distribution from very dry areas in eastern Maranh ã o to semideciduous seasonal forests in the far east of Pará and Tocantins, thus occurring in areas generally drier than the other species ( Figure 16 View FIGURE 16 ). The species is usually represented by shrubs to small trees 2–12 meters tall and, less commonly by medium trees of 12–20 meters, with two registered individuals reaching 25 meters in height (R. Vilhena 1002 ; G. Pereira-Silva 9771), presenting, therefore, a size considerably smaller than the species M. elatum and M. parviflorum , a little smaller than M. fluminense and similar to M. excelsum .
A specimen without indication of location or date of collection (J.L.S. Lima 1812) contains the location name “Petrolina/ Pernambuco ” on its label, a state with no record of occurrence of the genus. However, such location apparently refers to the institute to which the collector belonged (HSTA) and not the collection. The sites of closer collections from the same collector (J.L.S. Lima 1811; 1813) refer to northwestern Bahia, an area with an old record of the species. However, due to the absence of further information, we could not identify whether such a specimen would have been collected in this region. Another individual of the species is misquoted for an area considerably distant from the main distribution already mentioned (C. Correia 536). Although the label indicates the state of Paraíba, the municipality, location and geographic coordinates (Oeiras, Morro da Cruz) indicate the state of Piauí, where the species is common. Thus, so far, the genus does not occur in Pernambuco, Paraíba or any coastal area of Northeastern Brazil.
Its closely related genus Dicorynia has samaroid fruits similar to those of Martiodendron and which are known to be consumed by several genera of birds of the Psittacidae family ( Jesel 2005). Considering that M. mediterraneum has vernacular names such as “macaw bean” and “parrot’s perch”, it can be conjectured that native parrots also consume its fruits. The flowers are visited by bees (Oliveira, F.C.S. 346).
Etymology: —The species received the epithet from the Latin mediterraneus, which means “from the interior”, referring to the species occurring relatively far from the northeast coast, in savannas and semideciduous forests of the interior. Koeppen & Iltis (1962) have already commented on the misfortune of this name given by Martius, leading to possible confusion of this Brazilian species with taxa from the Mediterranean Sea.
Phenology: —It usually blooms from February to April, more rarely from December to June, with the apex of flowering in April; fruit from April to October, with the peak of fruiting in July.
Uses: —The medicinal use of the species is mentioned in Piauí; however, no information was found on which part of the plant would be used or the disease treated (F.C.S. Oliveira 127). Popular knowledge about the exceptionally hard wood is evident with the widespread use of vernacular names such as “axe-breaker”. Its wood is apparently little used. The fruits are used to feed goats (M.S.B. Nascimento 256) and the species is commonly mentioned as having great ornamental potential due to its abundant and beautiful blooms.
Conservation: —An EOO of 846,530 km ² was estimated for M. mediterraneum , being the second most distributed and, by far, the most common species of Martiodendron , with more than 150 observed specimens here, many of them collected recently. It is considered frequent in several regions, but is present in few protected areas such as Parque Nacional de Sete Cidades in Piauí. Despite scarce use mentions, its area of occurrence has suffered high rates of deforestation in recent years (INPE 2021). Fernandez et al. (2021) indicate a least concern status for the species based on an inaccurate distribution. Here, based on the aforementioned data and in IUCN criteria (2019), we also suggest a least concern category for M. mediterraneum but we emphasize the importance of more detailed studies on the recent deforestation impacts in the species population. Concerning its two varieties, M. mediterraneum var. mediterraneum has an estimated EOO of 438,360 km ², with similar considerations to the aforementioned species, including a suggested least concern category. As for M. mediterraneum var. concinnum , with only two specimens, about 80 km away from each other, its EOO could not be calculated but, based on IUCN criteria D (2019), we could suggest a preliminary critically endangered category for the variety, highlighting the urgent need for more studies and actions for its conservation. We emphasize that our recent expedition to its only region of occurrence was not successful in finding the taxon, highlighting its possible rarity.
Vernacular Names: —Banha-de-galinha, Catinga-de-porco, Costela-de-vaca, Costela-de-anta, Fava-de-arara, Fígado-de-galinha, Garapiá, Orelha-de-onça, Pau-de-arara, Pau-capoeira, Pau-ferro, Pihtyire (Indigenous name), Quebra-machado, Tachi-vermelho, Sucupira and Sucupira-preta. The name Garapiá is also used for Apuleia , a similar and closely related genus in Dialioideae . On the other hand, ironwood and quebra-machado are used for numerous other legume species, including Dialium , an also similar and closely related genus in Dialioideae .
Nomenclatural Comments: — Martiusia parvifolia , here considered synonymous of Martiodendron mediterraneum , has in its description, by Bentham (1840), only the collector and the place of collection (Gardner in Piauí). Bentham (1870) mentions the number 2149, also mentioned by Koeppen & Iltis (1862), however, they did not indicate the herbarium where the material is located and many duplicates are available in numerous institutions, so it has become necessary its lectotypification. We choose a material from the Kew herbarium, probably observed by Bentham (1840) for his description of the species. Among the five duplicates deposited in this herbarium, we selected the one with flowers that contained the stamp of the Herbarium Benthamianum (K000056164).
As for the type of Amphymenium mediterraneum , basionym of Martiodendron mediterraneum, Koeppen & Iltis (1962) mentioned three Martius specimens at herbarium M as three possible different collections (Martius 73, 74, and 75), being the number 73 chosen by Koeppen & Iltis as lectotype. The authors even point out that these three numbers would not be collection numbers, but old herbarium numbers. However, looking at such materials, only 73 has such a number written on the specimen. The other two specimens do not have numbers and we do not know how the authors discovered the aforementioned numbering. The location descriptions on all three materials are identical, so that we infer that these three materials are actually duplicates of the same collection and we characterize the materials called by the authors “74” and “75” as possible isolectotypes. As none of the three materials has a year of collection (only the months of March to April are mentioned in one of them), studying the trajectory followed by the naturalist in Brazil from 1817 to 1820, we were able to conclude that the probable collection date in the Serra da Itiuba, Bahia, was between March and April 1819.
Biogeographical Comments: —Two main populations were identified within this species. They are largely geographically isolated, with one occurring in northeastern and northern Brazil and one occurring in a small area of southeastern Brazil, both in Cerrado or, rarely, ecotone areas. The presence of several morphological differences between these two groups of plants and their occurrence in different regions enabled their taxonomic separation. Because these variations are a matter of degree, with some overlapping, we consider the distinction of these taxa to be two different varieties within M. mediterraneum . Although the distance between their areas of occurrence implies a low possibility of gene flow between populations of both varieties, we conjecture that this separation is still recent and that the two groups of individuals are still in the process of speciation.
Taxonomic Comments: — Martiodendron mediterraneum are generally smaller trees than M.elatum , M.fluminense and M. parviflorum . It differs from all other species in the genus by its environment of occurrence. It usually has more leaflets than M. excelsum and M. parviflorum and fewer leaflets than M. elatum ; the terminal ones usually narrower than in M. excelsum and generally shorter than in M. elatum and M. parviflorum . Its leaflets often have a wavy margin and lustrous surface, differing from M. excelsum and M. parviflorum . It has shorter axillary buds than M. elatum and generally longer axillary buds than M. parviflorum . Its inflorescences are usually smaller than in M. excelsum and M. parviflorum . It differs from M. fluminense by its often larger and solely terminal thyrsoid inflorescences and fewer stamens and from M. parviflorum by its straight floral buds. Its anthers are glabrous, differing from M. excelsum and M. parviflorum . It differs from M. excelsum by its fully pubescent gynoecium. Its fruits are generally smaller than M. elatum , M. fluminense , and M. parviflorum ( Table 1 View TABLE 1 ).
Identification key to the varieties of Martiodendron mediterraneum
1. Leaves (10.8–)12–21(–25) cm long, the maximum distance between leaves (1–)2–6(–12) cm forming lax branches; terminal leaflets generally ovate, elliptical or oblong, rarely narrowly oblong, base almost always cordate to truncate, rarely obtuse, (4–)5– 9.5(–11) × (1.7–)2–3.7(–5.5) cm; in Maranh ã o, Piauí, Tocantins, and extreme eastern Pará .............................................................. ............................................................................................................................ Martiodendron mediterraneum var. mediterraneum View in CoL
- Leaves 10–10.5 cm long; the maximum distance between leaves 0.5–1.5(–2) cm forming more congested branches; terminal leaflets narrowly oblong, base obtuse, 2.5–6 × 1.1–1.6 cm; in northern of Minas Gerais ................................................................... .................................................................................................................................. Martiodendron mediterraneum var. concinnum
| M |
Botanische Staatssammlung München |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Martiodendron mediterraneum (Mart. ex Benth.) R.C. Koeppen
| Falcão, Marcus José De Azevedo, Torke, Benjamin M., Garcia, Gabriel Santos, Silva, Guilherme Sousa Da & Mansano, Vidal De Freitas 2023 |
Martiodendron mediterraneum (Mart. ex Benth.) R.C. Koeppen
| Mart. ex Benth. 1962: 203 |