Martiodendron excelsum (Benth.) Gleason, 1935
|
publication ID |
https://doi.org/10.11646/phytotaxa.578.1.2 |
|
DOI |
https://doi.org/10.5281/zenodo.7542598 |
|
persistent identifier |
https://treatment.plazi.org/id/B43787C8-7C35-FFCA-FF50-76734766F818 |
|
treatment provided by |
Plazi |
|
scientific name |
Martiodendron excelsum (Benth.) Gleason |
| status |
|
Martiodendron excelsum (Benth.) Gleason View in CoL View at ENA . Phytologia 1(3):141 (1935).
≡ Martiusia excelsa Benth. View in CoL in Hook. Journal of Botany, being a second series of the Botanical Miscellany 2: 84 (1840). ≡ Martia excelsa Benth. View in CoL in Hook. Journal of Botany, being a second series of the Botanical Miscellany 2: 146 (1840).
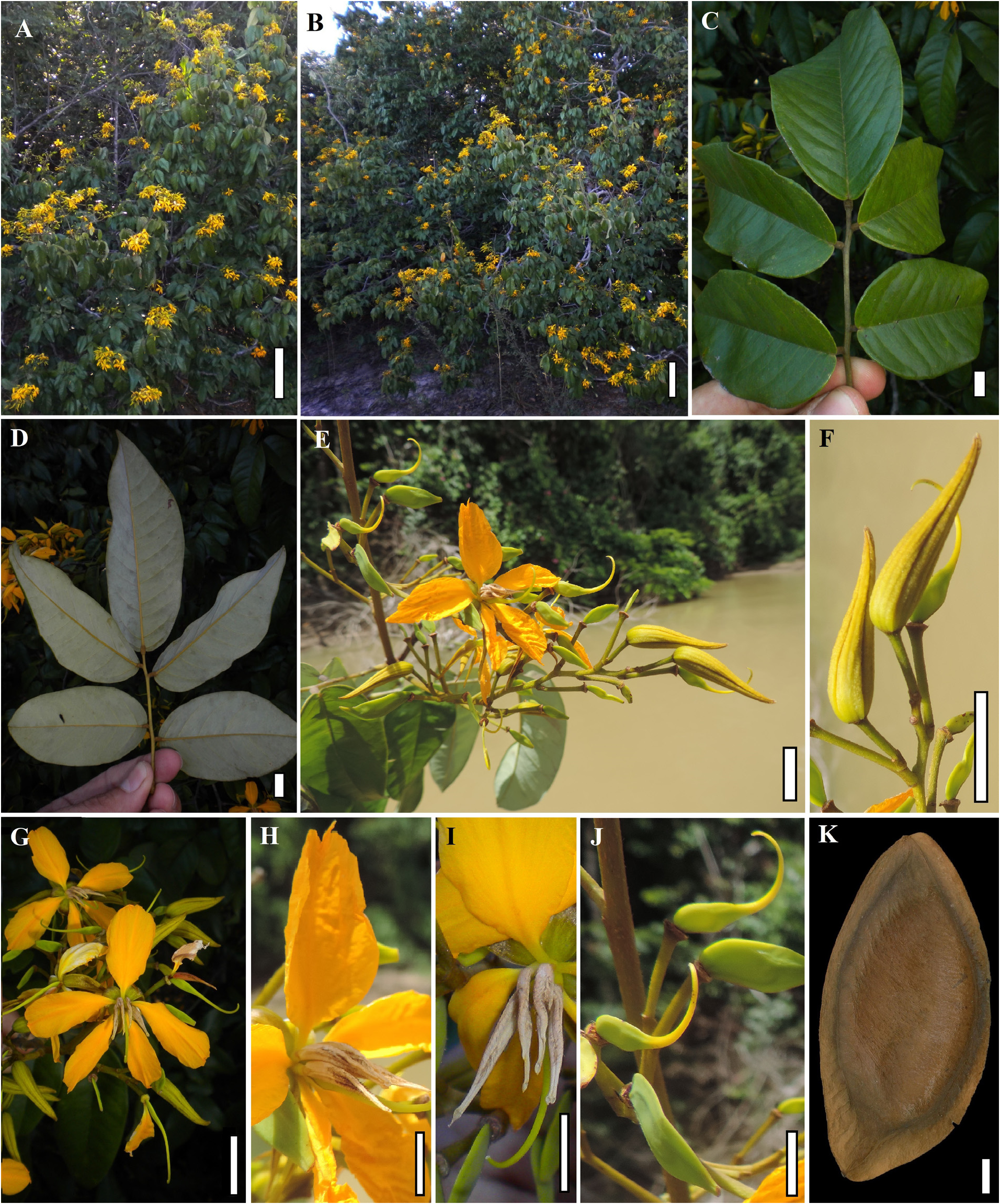
Type:— Guiana: Quitaro ( Kwitaro ) River , [X-XII-]1837, Schomburgk, R. H. 589 ( Lectotype, designated by Koeppen & Iltis [1962: 200]: K barcode K000264610 !; Isolectotypes: BM!; BR!; E!; F!; GH!; NY!; P!; US!). ( Figures 1–2 View FIGURE 1 View FIGURE 2 ) .
Shrubs to small trees 1–15 m tall, rarely up to 25 m, up to 30(–55) cm in diameter, usually without buttresses. Leaf rachis (1.5–)5.5–14.5(–17) cm long; petiole 1.5–3.2 cm long; petiolule 3–5 mm long; leaflets (3–)5–6(–7), rarely unifoliolate leaves at the base of the inflorescences, the blades chartaceous, margins commonly revolute, the terminal ones (7–)8–12(–19) × (3.2–)5–9(–11) cm, 1.4–2(–2.6) times longer than wide, elliptical to ovate, more rarely oblong, apex cuspidate to acuminate to rounded, base usually rounded to truncate or cordate, less commonly obtuse; axillary buds elliptic to oblong in outline, (4–)5–6.7 × 1.9–2.7 mm, apex acuminate to cuspidate. Inflorescences thyrsoid, distichous, terminal, with elongated primary and secondary axes from which cymes are formed, (7–)15–25(–40) × 6–20(–27) cm. Flower buds 1.5–3 cm long, straight, apex almost always straight, acute to acuminate; sepals 1.5–3 × 0.1–0.4 cm, one of them generally narrower than in other species, sometimes the abaxial sepal patent and the others reflexed; petals 0.9–2(–3) × 0.7–1.5 cm; stamens 4 or 4+1 abaxial staminode, rarely 5 stamens, the abaxial one less-developed than the others, anthers (0.8–)1.2–2 × 0.1–0.25 cm, densely pubescent, the pubescence generally concentrated in the middle portion of the anther; carpel 5–7 × 2–3 mm, laterally glabrous, pubescent along the abaxial suture; style 5–12 mm long. Fruits almost always elliptical, sometimes slightly asymmetrical, 5.5–9(–10) × 2.8–5 × 0.3–0.8 cm, 1.7–2.2 times longer than wide, glabrous, greenish or yellow to orange, sometimes with pink or red spots along the sutures when mature, wings each 3–7 mm wide in the middle portion of the fruit, seminiferous nucleus occupying most of the fruit, ca. 6–10 times wider than the widest of the two wings in the middle portion of the fruit.
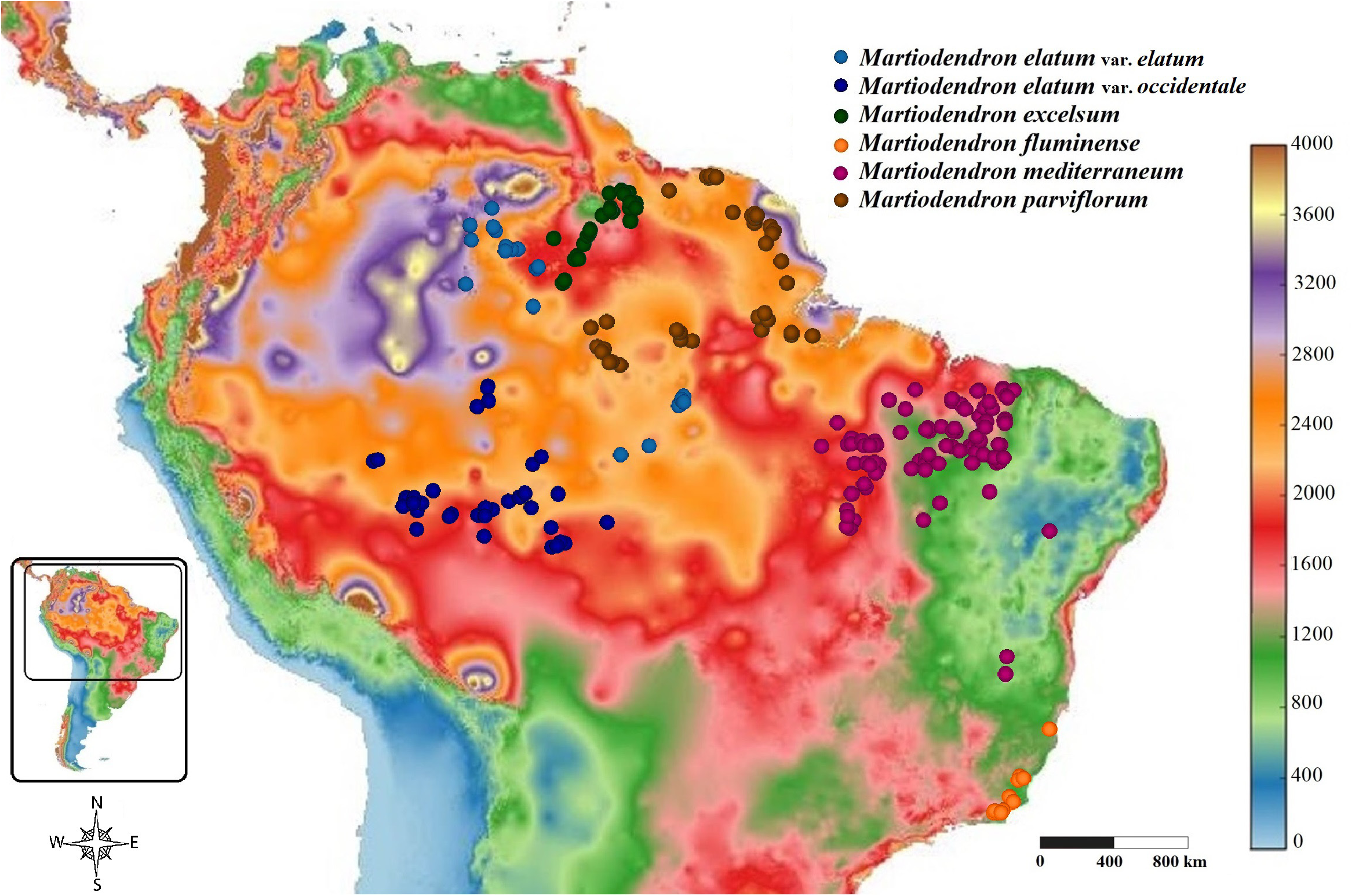
Distribution, Habitat and Ecology: —The species occurs in Guyana and in the Brazilian state of Roraima ( Figure 15 View FIGURE 15 ), in the basins of the Branco and Essequibo Rivers. It may also occur in Venezuela, near to the Brazilian and Guayanan borders. The species is almost always found in campinaranas, varzeas and riverbank forests, and rarely in upland savannas but always close to rivers, on sandy or sandy-clay soils. Ducke (1948) considered it one of the most frequent and characteristic species of the marginal forest of the Rio Branco in Roraima. In Guyana, the species is dominant along the edges of Essequibo River in the Iwokrama Reserve ( P. Mutchnick 834). Borges et al. (2014) reported M. excelsum occurring in Mato Grosso, where only M. elatum occurs, but the authors did not cite any specimen.
The species is shorter and occurs in considerably drier areas than the other Amazonian species ( Figure 16 View FIGURE 16 ). It is often found as shrubs to small trees 1 to 6 m tall. Median trees with 6–15 m tall are common, rarely reaching a maximum of 25 m tall. Although Silva et al. (2005) and Koeppen & Iltis (1962) gave heights of 30–50 m and 28–33 m, respectively, and the specific epithet invokes a large tree (see etymology); we found no specimens in situ or in herbaria with label data indicating a stature greater than 25 m. However, we examined all of the specimens cited by Silva et al. (2005) and all but three of those cited by Koeppen & Iltis (1962); indeed, the total number of specimens of the species examined for the present study was almost triple the number mentioned by Koeppen and Iltis. Perhaps this discrepancy is due to misinterpretation of the tree height given in feet rather than meters on many older specimens. Because this was the first species of the genus to be described, the idea of height expressed by its name should not be taken as compared to other species of the genus.
Specimen label data ( Lewis, G.P. 1607) and personal observation indicate that fruits of Martiodendron excelsum are often found on the banks of rivers and that they float in water due to an airspace in the inner chamber of the samara. These observations, combined with the relatively reduced samara wings of the species relative to other members of the genus, indicate an evolutionary shift away from anemochoric dispersal, which presumably is the dominant form of seed dispersal in the other species of the genus, toward hydrochoric dispersal. Transitions to hydrochoric dispersal via floating fruits are postulated for several other taxa of Dialioideae found in seasonally inundated riverine forests and/or close to rivers, including some species of Dialium , Dicorynia , and Apuleia (Falc ã o et al., 2016; 2020b; 2022; in prep). Other examples of mainly anemochoric groups with shifts towards hydrochory are found in other subfamilies of Leguminosae like in the genera Machaerium Pers. and Pterocarpus Jacq. , of subfamily Papilionoideae ( Lozano & Klitgaard 2006; Schley et al., 2021).
Etymology: —The species was named by Bentham (1840) from the Latin excelsus, a reference to a large size, a similar meaning to the epithet of M. elatum . However, M. excelsum is generally a much smaller tree than M. elatum , M. fluminense , and M. parviflorum .
Phenology: — Martiodendron excelsum usually blooms from November to March, more rarely from September to July; it usually bears fruit from January to April, more rarely throughout the year.
Uses: —Notes on W. Rodrigues 688 indicate that the seeds of the species are consumed as an ingredient of tapioca preparations in Roraima.Although the wood is extremely hard and presumably durable, similar to that of other species, we found no records of its use. Like other species of the genus, M. excelsum holds great potential for use as ornamental due to its profuse and showy flowering.
Conservation: —An EOO of 80,832 km 2 was estimated for M. excelsum , indicating that the species should be assigned to IUCN Red List category of Least Concern, but the species is restricted to Guyana and Brazil, in two river basins. Only the collections by J.M. Pires 14386 (in 1974) and P. Mutchnick 834 (in 1996) indicate that the species is common in these areas. Of the 46 specimens observed here, only 8 were collected after the 2000s; However, the species is known to occur in relatively few conservation areas, including Viruá National Park and the Caracaraí Ecological Station in Brazil and Iwokrama Forest in Guyana. Moreover, the species appears to be restricted to riverine areas, which makes the species highly vulnerable not only to deforestation, which is more intense in areas close to rivers due to ease of accessibility, but also to flooding and changes in hydrology caused by hydroelectric projects, mining and other types of development ( Assahira et al., 2017). There is a planned project to build a hydroelectric plant on the Branco River (UHE Bem Querer), which would result in the flooding of a large area of riparian habitat along the river, where the distribution of M. excelsum in Brazil is concentrated ( Brazil, EPE 2020; ICMBio 2013; present work). Considering these factors, we recommend that the species be placed in the Near Threatened category.
Vernacular Names: —Muirapixuna, Muirapichuna, Muriapichuna, Tapioqueira ( Brazil) and Tatabuballi ( Guyana).
Nomenclatural Comments: — Koeppen & Iltis (1962) designated as lectotype of Martiodendron excelsum the duplicate of Schomburgk 589 housed in K. Bentham (1840) cited two Schomburgk collections ( 49 and 589) in the protologue of the basionym, Martia excelsa . However, it is well documented that two different numbers, belonging to the number series of the brothers Robert and Richard Schomburgk, who collected both together and apart in Guyana between 1835 and 1844, were sometimes given to the same gathering ( van Dam 2002; Alexander 2011). Indeed, a sheet in P (barcode P 02142572) has a label bearing both numbers, 49 and 589. However, a tag is attached to the specimen mounted on the sheet with only the number 589. Moreover, with examination of the other duplicates, as well as the two explorers’ accounts of their journeys in their personal diaries and collection logs ( van Dam 2002; Alexander 2011), we concluded that the two numbers represent different gatherings, both made by Robert Schomburgk. The gathering labelled as no. 49 was collected along the upper Essequibo, near its meeting with the Rupununi River, probably between December of 1835 and March of 1836, while the gathering labelled as no. 589 was collected along the Kwitaro River, probably between October and December of 1837. Another Schomburgk collection, no. 235/313 is a third separated gathering.
Taxonomic Comments: —In the context of the genus, Martiodendron excelsum mainly differs from the other four species for being shrubs to small trees with a preference for frequently inundated riverine forests, having fewer leaflets per leaf, the terminal ones generally wider, laterally glabrous gynoecium, greenish to yellowish to orange fruits, elliptical, shorter, with smaller wings and seminal nucleus occupying most of the fruit. Together with M. parviflorum , M. excelsum has pubescent anthers, which differ from the other three species, but the pubescence is more evenly distributed along the anther in M. parviflorum and generally more concentrated in the middle portion of the anther in M. excelsum . It also differs from M. fluminense by its thyrsoid inflorescences and four stamens (vs. cymose inflorescences and five stamens) and from M. parviflorum by its longer axillary buds and straight and longer floral buds ( Table 1 View TABLE 1 ). The sepals of M. excelsum are somewhat more heteromorphic than the other four species of Martiodendron , with the character of one sepal narrower than other, common in the genus, being generally more evident in M. excelsum .
Ducke (1935) suggested that unlike other species of the genus that have red- or purple-maturing fruits, M. excelsum always has greenish fruits. However, we encountered specimens with label data describing yellow fruits marked with pink or orange ( G.P. Lewis 1607; K.M. Redden 1169). Nevertheless, fruit color does seem to be a helpful character in distinguishing M. excelsum from other species of the genus. Some features found by Silva et al. (2005) as the presence of a hypanthium’ and only four sepals and four petals in M. excelsum were not observed here despite analyzing all specimens cited by Silva et al. (2005).
Selected List of Additional Specimens Examined: (20 of 45 analyzed specimens): —BRAZIL: Roraima: Beira do Rio Branco , entre as fazendas Capela e Bom Intento, 7-XI-1951, Black , G. A. 51-14057 ( IAN; NY; P; R; US); Jarú , XII-1913, Kuhlmann, G. 58 ( RB); Kuhlmann, G. s.n. RB2817 ( RB; US); margens do rio Caname nas proximidades das praias do Camari. Arbusto de 3 m, 18-XI-1977, Rosa , N. A. 1552 ( RB; MG; NY); perto da boca do rio Ajarani com o Rio Branco , 28-IV-1974, Pires, J. M. 14386 ( IAN; MG); Boa Vista: Banho do Curupira, margem direita do rio Cauamé GoogleMaps , 2°52’18”N 60°40’20”W. Árvore de +- 5 m de altura e +- 55 cm de diâmetro, 9-II-2004, Barbosa , R. I. 86 ( INPA); Rio Branco, margem alta da boca do Cauamá. Árvore pequena, 10-IX-1943, Ducke, A. 1389 ( IAN; MG; NY; R); Caracarai Road , 58 km. S. of Boa Vista. Tree, 16 m × 20 cm, 31-I-1969, Prance, G. T. 9518 ( INPA; MG; NY; R; US); Caracaraí: Parque nacional do Viruá GoogleMaps , entre kms 5 e 7, 2°44’N 62°05’ W, campinarana, Árvore de 15 m × 30 cm DAP, 14-III-2007, Cid Ferreira, C. A. 12986 ( INPA); Rio Branco , 22-VII-1939, Ducke, A. s.n. RB24186 ( RB; US); Rio Branco GoogleMaps , margem esquerda, ilhas inundáveis e igapós, 0°36’14”N 61°35’43”W. Arvoreta, 11 m, 20-III-2012, Martinelli, G. 17381 ( ESA; RB); indicated as cultivated in Rio de Janeiro, Brazil , Glaziou, A. 13428 (P); GUYANA: Potaro-Siparuni: Iwokrama Forest Reserve: Siparuni River, between Whitewater camp and Turtle mountain , 4°48’56”N 58°51’1”, riverside. Tree , 25 m tall, 20 cm DBH, 7-XI-2002, Redden, K. M. 1169 ( U; US); Iwokrama Mts. Kurupukari base camp 4°40’N 58°40’W. Tree 10 m, 29-XI-1995, Clarke, D. 685 ( NY; US); Esequibo River GoogleMaps , kurupukari falls, 4°40’N 58°40’W, riverine forest and islands of sandy soils. Tree, 11-XII-1994, Mutchnick, P. 645 ( NY; U; US); Kurihi rapids. Tree 40’, 8’’ diam., 31- I-1944, Forest Department of British Guiana 4299 ( NY); U.Takutu-U. Esequibo: Upper Esequibo / Rupununi River . Tree 40-50 ft, XII-1835 to III-1836, Schomburgk, R. 49 ( E; F; GH; K; P); SE of Karasabai to Yourora Creek GoogleMaps , 4°1’N 59°31’W, Tree +- 5-15 m × +- 15-20 cm DBH, 9-III-1990, McDowell, T. 2189 (NY; US); U. Demerara: probably 1867, Appun, C. F. s.n. P3207077 ( P); Unknown locality in Guyana: 1843, Schomburgk 235/313 ( K; P).
List With Summary Data of Additional Specimens Examined: (25 of 45 analyzed specimens):— BRAZIL: Roraima: Silva, M.T. 4552 (MG; NY); Ule, E. 7772 (K; MG); 7605 (MG); Rodrigues, I.A. 524 (IAN); Rodrigues, W. 688 (INPA; MG; NY; US); Fróes, R.L. 22930 (IAN; MG; US); Lewis, G.P. 1607 (INPA; K; NY); Scḩtz Rodrigues, R. 1618 (RB); Rosa , N.A. 1422 (INPA; MG; NY); Perdiz, R.O 1106 (MIRR); Toledo, C.A.P. 430 (RB); Ducke, A. s.n. RB751801 (RB); Pires, J.M. 14507 (IAN; INPA; MG; NY; RB; US); GUYANA: Potaro-Siparuni: Mutchnick, P. 834 (MO; NY; US); 543 (NY; U; US); Clarke, D. 1358 (NY; US); Mori, S. 24350 (NY; U; US); Atkinson, D.J. 82 (US); Hoffman, B. 1366 (INPA; NY; U; US); Redden, K.M. 5080 (NY; US); Forest Department of British Guiana 4440 (NY) U.Takutu-U. Esequibo: Hoffman, B. 3859 (F; NY; US); Knapp, S. 2788 (MO); Hoffman, B. 1233 (F; MO; NY; US); U. Demerara: Schomburgk 119 (P).
| G |
Conservatoire et Jardin botaniques de la Ville de Genève |
| A |
Harvard University - Arnold Arboretum |
| IAN |
Embrapa Amazônia Oriental |
| NY |
William and Lynda Steere Herbarium of the New York Botanical Garden |
| P |
Museum National d' Histoire Naturelle, Paris (MNHN) - Vascular Plants |
| R |
Departamento de Geologia, Universidad de Chile |
| US |
University of Stellenbosch |
| RB |
Jardim Botânico do Rio de Janeiro |
| N |
Nanjing University |
| MG |
Museum of Zoology |
| J |
University of the Witwatersrand |
| M |
Botanische Staatssammlung München |
| I |
"Alexandru Ioan Cuza" University |
| INPA |
Instituto Nacional de Pesquisas da Amazonia |
| S |
Department of Botany, Swedish Museum of Natural History |
| T |
Tavera, Department of Geology and Geophysics |
| C |
University of Copenhagen |
| ESA |
Universidade de São Paulo |
| U |
Nationaal Herbarium Nederland |
| E |
Royal Botanic Garden Edinburgh |
| F |
Field Museum of Natural History, Botany Department |
| GH |
Harvard University - Gray Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Martiodendron excelsum (Benth.) Gleason
| Falcão, Marcus José De Azevedo, Torke, Benjamin M., Garcia, Gabriel Santos, Silva, Guilherme Sousa Da & Mansano, Vidal De Freitas 2023 |
Martiodendron excelsum (Benth.)
| Benth. 1935: 141 |