Trypanosoma madeirae, Barros & Lima & Schubach & Teixeira, 2019
|
publication ID |
https://doi.org/ 10.1016/j.ijppaw.2018.12.009 |
|
DOI |
https://doi.org/10.5281/zenodo.11042721 |
|
persistent identifier |
https://treatment.plazi.org/id/B56587E6-F038-FF8B-F612-FBAF59E9FC7A |
|
treatment provided by |
Felipe |
|
scientific name |
Trypanosoma madeirae |
| status |
|
5.1. Phylogenetic positioning of Trypanosoma madeirae n. sp
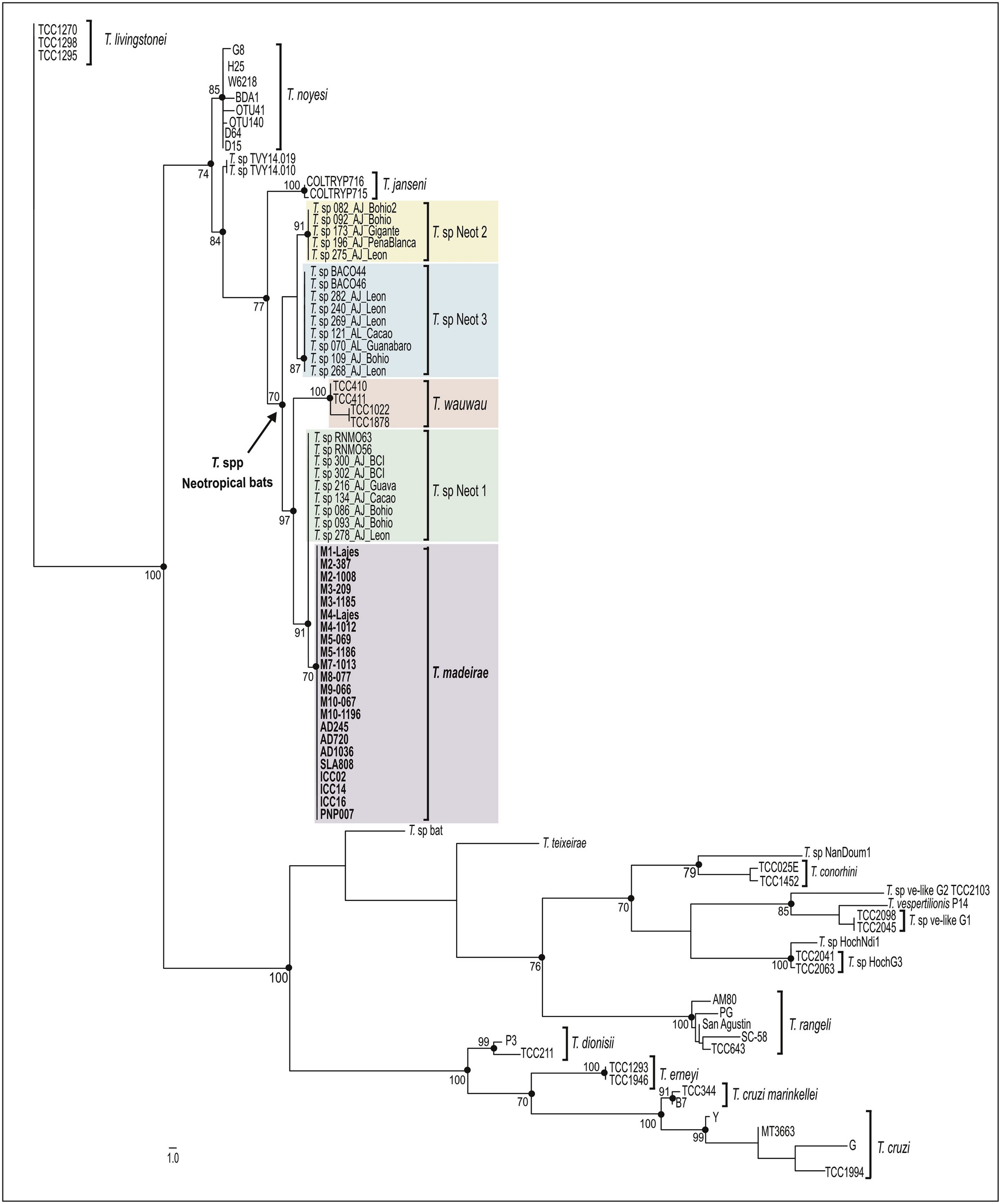
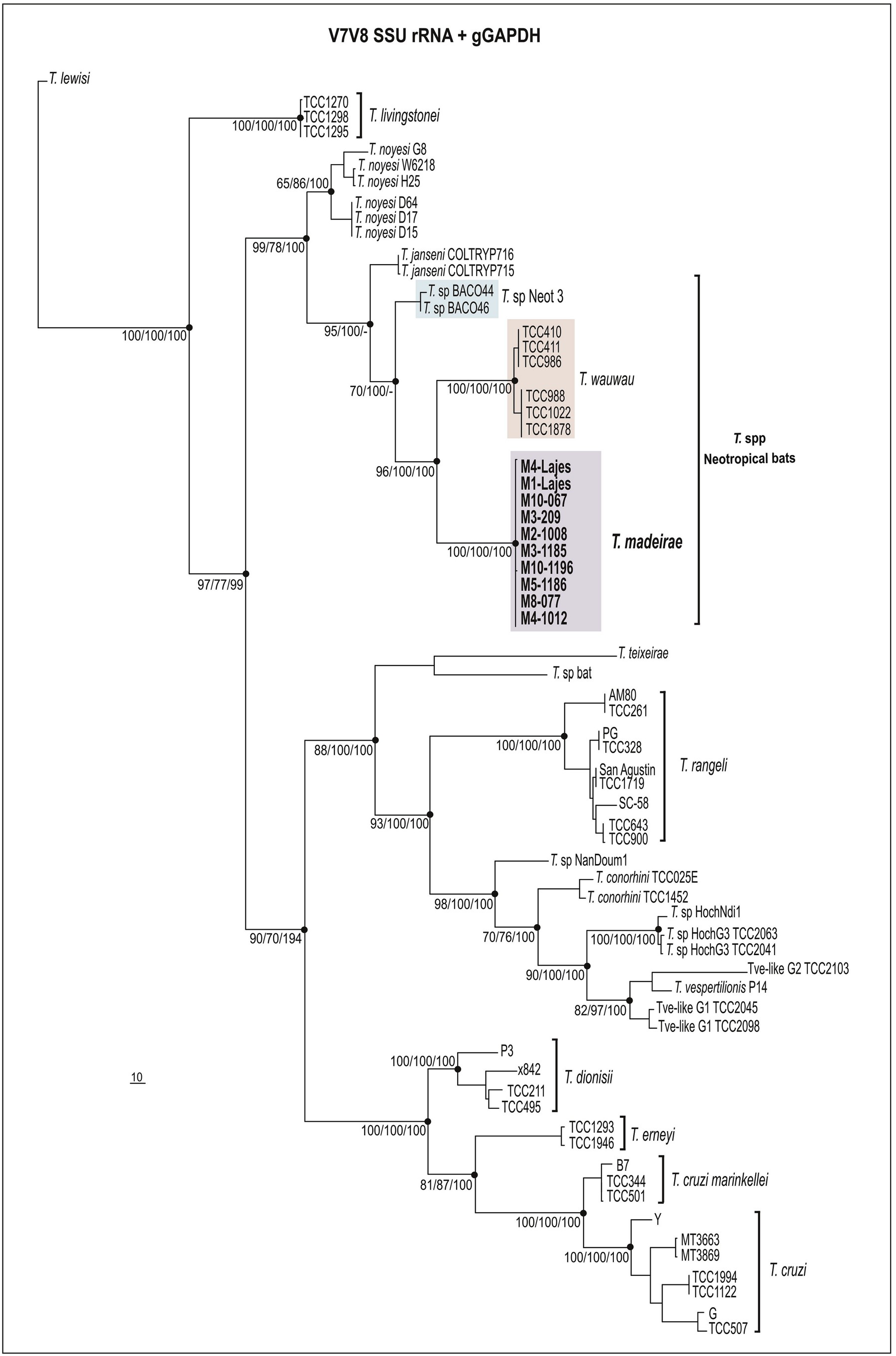
In the present study, we described Trypanosoma madeirae n. sp isolated from the hematophagous bat D. rotundus captured in the Atlantic Forest biome, Rio de Janeiro, Southeast Brazil. To date, this species was identified only in D. rotundus species. Altogether, phylogenetic inferences and degrees of sequence divergences based on V 7 V 8 SSU rRNA and gGAPDH sequences strongly support T. madeirae placed within the T. cruzi clade ( Figs. 2 View Fig and 3 View Fig ), similar to most bat trypanosomes described to date. Thus, our findings provide additional support to the ‘bat-seeding’ hypothesis for the origin of the species of this clade (Hamilton et al., 2012). Trypanosoma madeirae n. sp. clustered with other trypanosomes from phylostomid bats in the Neobat phylogenetic lineage, which comprises closely related trypanosomes distributed in the clades Neotropic 1, 2, and 3. Each clade is formed by sequences obtained from bat blood samples representing a single species waiting for a formal taxonomic description. Trypanosoma madeirae is very closely related to T. sp Neotropic 1, so far detected in Trachops cirrhosus and Artibeus jamaicensis (phyllostomids) in Brazil and Panama. The high similarity of SSU rRNA sequences shared by both T. madeirae and T. sp Neot 1 suggests that these two trypanosomes likely represent very closely related species or different genotypes of T. madeirae , but an answer to this question requires comparative analyses using the more polymorphic gGAPDH sequences. However, T. madeirae n. sp. was clearly separated from T. wauwau , the only named species of trypanosome within the Neobat lineage ( Figs. 2 View Fig and 3 View Fig ).
The Neobat lineage also harbors T. wauwau (Lima et al., 2015a) , the only species of this lineage obtained in culture and formally described before T. madeirae . The trypanosomes positioned basal to this lineage were T. janseni from a Neotropical marsupial (Lopes et al., 2018), T. noyesii from Australian rodents and marsupials (Hamilton et al., 2012b; Botero et al., 2016), and one unnamed trypanosome of lemurs from Madagascar known just by a small DNA sequence (Larsen et al., 2016) ( Figs. 2 View Fig and 3 View Fig ).
In addition to T. madeirae , T. cruzi , T. c. marinkellei, T. dionisii , T. rangeli , and Trypansoma spp Neot 1, 2, and 3 have been molecularly identified in D. rotundus ( Brazil and Venezuela). However, differing from T. madeirae , which was so far detected exclusively in D. rotundus , these trypanosomes have been detected in a range of bat species (Cavazzana et al., 2010; Cottontail et al., 2014; Ramírez et al., 2014; Pinto et al., 2015). Unfortunately, T. pessoai , a species previously reported in D. rotundus in Brazil (Deane and Sugay, 1963; Deane et al., 1978; Molyneux, 1991; Vilar et al., 2004), is not available for molecular comparison with T. madeirae .
5.2. Morphological and biological characterization of T. madeirae n. sp
The flagellates from log- and stationary-phase cultures were examined by light morphology. The typical epimastigote forms of T. madeirae at log-phase cultures were slender flagellates with a near central nucleus, small lateral kinetoplast, and an under-developed undulating membrane. Both epimatigotes and trypomastigotes of T. madeirae differ from those of T. cruzi , T. dionisii , and T. rangeli (Maia da Silva et al., 2009; Lima et al., 2012).
By taking into account its phylogenetic positioning in the Neobat lineage, we compared behavioral and morphological features of T. madeirae with those described for T. wauwau , the only closely related species that are available in culture and was previously isolated in culture and morphologically characterized. Both epi- and trypomastigote cultured forms of T. wauwau markedly differ from those observed in cultures of T. madeirae (Lima et al., 2015a) . We demonstrated that T. madeirae can survive at 37 ̊C and enter murine macrophagic cells, probably internalized by phagocytosis, but it is unable to survive inside these cells. Similar behavior was observed in the closely related T. wauwau using monolayers of monkey LLC-MK2 cells (Lima et al., 2015a). In contrast, all bat trypanosomes of the subgenus Schizotrypanum , such as T. cruzi , T. dionisii , and T. erneyi , invade, differentiate and replicate within macrophages, LLC-MK2, and other mammalian cells (Baker et al., 1971; Cavazzana et al., 2010; Lima et al., 2012; Maeda et al., 2012; Espinosa-Álvarez et al., 2018).
The complement system, a key component of innate immunity, plays a very important role as the first line of defense against trypanosomes (Lidani et al., 2017). The epimastigotes of T. madeirae are susceptible to lysis by human complement system, similar to epimastigotes of T. cruzi , Trypanosoma desterrensis and T. dionisii , which are both species of the subgenus Schizotrypanum , whereas epimastigotes of T. rangeli are not lysed when incubated with fresh human sera (Schottelius et al., 1986; Steindel et al., 1998; Maeda et al., 2012). Is it well known that when describing new Trypanosoma species it is very challenging to find the right culture media to grow all stages, especially to induce transformation and growth of metacyclic trypomastigotes. Although we have been done some attempts to enhance metacyclic forms in cultures, T. madeirae always shows up low percentage of typical trypomastigote forms. These results may have influenced either macrophage infection rates and/or survival inside cell. It is important to consider that metacyclic trypomastigotes of T. madeirae , which were scarce even in stationary cultures, may exhibit differences regarding susceptibility to the human complement system. Differing from the complement-resistant metacyclic trypomastigotes of T. cruzi , metacyclic trypomastigotes of T. dionisii are susceptible to complement-mediated lysis (Maeda et al., 2012).
Previous studies showed that most cultured trypanosomes of the clade T. cruzi did not develop in triatomine bugs (Cavazzana et al., 2010; Lima et al., 2012, 2013, 2015b) T. cruzi and T. rangeli are so far the only species unquestionably cyclically transmitted by triatomines. Here, many attempts of infecting T. infestans with T. madeirae failed; the flagellates were completely destroyed in the digestive tract of the bugs after ∼15 days. Previous efforts of obtaining established experimental infection of Triatoma , Rhodnius and Panstrongylus species with T. c. marinkellei, T. dionisii , T. erneyi , and T. cruzi of the genotype TcBat have all failed, despite the ability of all these species to survive for many days in the digestive tract of the triatomines (Cavazzana et al., 2010; Lima et al., 2012, 2015b). Recently, T. c. marinkellei and T. dionisii were detected by PCR surveys in the digestive tract of Triatoma viticipes (Dario et al., 2017a,b), but colonization of the triatomine guts by these species was not demonstrated. In addition to the fact that T. cruzi is cyclicaly transmitted by a range of triatomines species, it was welldemonstrated that T. dionisii and T. vespertilionis are cyclically transmitted by cimicid bugs (Bower and Woo, 1982; Gardner e Molyneux, 1988; Espinosa-Álvarez et al., 2018). In addition, sand flies were incriminated as vectors of T. pessoai and T. leonidasdeanei to neotropical bats (Zeledón and Rosabal, 1969; Deane et al., 1978). The epidemiological and ecological data suggest that transmission of trypanosomes among bats should also occur through ingestion (during grooming) of their ectoparasites (flies, ticks, bugs, mites, and fleas) containing trypanosome infected blood meal (Cavazzana et al., 2010; Lima et al., 2012, 2013, 2015a; Barbosa et al., 2016; Dario et al., 2017a,b; Espinosa-Álvarez et al., 2018).
Therefore, vectors of T. madeirae and all other trypanosome species nested in the Neobat lineage are so far unknown. Many cave-dwelling hematophagous insects living together with D. rotundus , such as mosquitoes ( Culicidae ), sand flies (Phlebotominae), bat flies ( Nycteribiidae and Streblidae ), biting midges ( Ceratopogonidae ), bat bugs (cimicidae), fleas and ticks (Obame-Nkoghe et al., 2017), are all potential vector candidates. It is also tempting to speculate whether the transmission of T. madeirae , apparently specifically among D. rotundus , might be due to its social cooperative behavior of sharing blood meals that is regurgitated to feed starving bats (Wilkinson et al., 2016), thus allowing the transmission of this trypanosome specifically among bats of this species.
5.3. Phylogeography and host-parasite association
Notably, taking into account the large number of surveys of trypanosomes in Neotropical bats carried out using molecular methods, T. madeirae was exclusively found in D. rotundus ( Phyllostomidae ). This species was detected in 22 (14 cultures and 8 archived blood samples) out of 130 (78 captured in RJ and examined by hemoculturing and 52 from other regions screened by nested PCR) specimens of D. rotundus captured from Northern to Southern Brazil. The survey of trypanosomes in more than 1700 bats captured across South America (Cavazzana et al., 2010; Pinto et al., 2015; Lima et al., 2015a,b; Dario et al., 2017a,b; Dos Santos et al., 2017; Bento et al., 2018; Lourenço et al., 2018) did not reveal T. madeirae in more than 60 species of bats examined, even though most species examined belonged to Phyllostomidae .
Taken together, our findings support T. madeirae as a new species of trypanosome, so far exclusively found in D. rotundus . Vampire bats from wide geographical range (North to South) and distinct Brazilian biomes were found infected with isolates of T. madeirae sharing virtually identical V 7 V 8 SSU rRNA barcodes. However, without experimental cross-infections, strict host-restriction of trypanosomes cannot be warranted to any trypanosome species, even though relevant data have suggested important degrees of association between some trypanosomes and their bat hosts. This study demonstrated that T. madeirae may be a species more linked to vampire bats among other trypanosome species that also infect D. rotundus , even those that also nested in the lineage Neobats. The Neobat lineage also harbors T. wauwau , a species linked to Pteronotus spp. ( Mormoopidae ) reported in large surveys of bats in many countries from Central and South America (from Amazon to the Atlantic Forest) (Lima et al., 2015b; Da Costa et al., 2016). Recently, T. wauwau was reported for the first time in one phyllostomid bat of the genus Anoura in Minas Gerais, Brazil (Pegorari et al., 2018). Previously, bats of Anoura captured in different biomes were found infected with T. dionisii (Cavazzana et al., 2010; Dario et al., 2017).
Supporting the link between D. rotundus and T. madeirae , other species of trypanosomes, T. dionisii and T. wauwau , were identified in bats sharing shelters with D. rotundus (Cavazzana et al., 2010; Lima et al., 2015). Interestingly, although vampire bats often shared shelters with other bat species, they generally hung separately (Delpietro et al., 2017). The frequent contact between blood meal of D. rotundus and wild and domestic animals (Johnson et al., 2014), and even humans may favor interspecific transmission of T. madeirae . Host switching appears to be a common process allowing for the expansion of host ranges of trypanosomes nested in T. cruzi clade, a process likely mediated by cimicid/triatomine vectors by which the generalist T. cruzi and T. rangeli most likely originated (Hamilton et al., 2012; Lima et al., 2012; Espinosa-Álvarez et al., 2018).
The lack of trypanosome geographical structure suggested a constant flow of bats carrying T. madeirae . This hypothesis is consistent with studies demonstrating that young males of D. rotundus systematically disperse to new colonies. Colonies of D. rotundus , with a longevity up to 16 years, can be large (> 300 bats) and in the absence of environmental disturbances adults spend most of their lifetime in the same or neighboring colonies, while young males migrate to more distant new colonies (Martins et al., 2009; Johnson et al., 2014). Successive dispersion of D. rotundus likely allowed for interchange and dispersion of their trypanosomes. The very interesting and apparent strong association of T. madeirae with D. rotundus must be further confirmed by more comprehensive surveys of trypanosomes from D. rotundus , other hematophagous bats, and bats of many other species and families, using more sensitive and effective methods suitable for unraveling the full repertoire of trypanosomes harbored by bats.
| V |
Royal British Columbia Museum - Herbarium |
| SSU |
Saratov State University |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.