Amnirana parva, Griesbaum & Jongsma & Penner & Kouamé & Doumbia & Gonwouo & Hillers & Glos & Blackburn & Rödel, 2023
|
publication ID |
https://doi.org/10.11646/zootaxa.5254.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:BF80CD4F-BEC8-4543-9172-47AE8F382E31 |
|
DOI |
https://doi.org/10.5281/zenodo.7732589 |
|
persistent identifier |
https://treatment.plazi.org/id/BA636469-FFED-C57C-FF38-FF74CEA8FF77 |
|
treatment provided by |
Plazi |
|
scientific name |
Amnirana parva |
| status |
sp. nov. |
Amnirana parva sp. nov.
Lesser White-Lipped Frog
Figs. 5–7 View FIGURE 5 View FIGURE 6 View FIGURE 7
Holotype. ZMB 88458 View Materials (field #: RG27 , GenBank #: 16S: OQ400914 ), adult male, Liberia, Krahn-Bassa Proposed Protected Area , 5°39′02.1′′N, 8°39′05.0′′W, 28 March 2018, leg. M.-O. Rödel & J. Glos. GoogleMaps
Paratypes. Twelve males , ten females: ZMB 71273 View Materials (field #: MOR ANK 15 , GenBank #: 16S: MG552248 ), adult female, Ghana, Ankasa National Park , 5°16′38.5212′′N, 2°38′41.8812″W, 5 August 2005, leg. A.C. Agyei & A. Hillers GoogleMaps ; ZMB 71274 View Materials (field #: MOR OWS 28 , GenBank #: 16S: MG552262 ), adult female, Ghana, Owabi Wildlife Sanctuary , 6°44′50.5212″N, 1°42′11.6388″W, July 2005, leg. A.C. Agyei & A. Hillers GoogleMaps ; ZMB 71276 View Materials (field #: MOR LE 11 , GenBank #: 16S: MG552287 ), adult female, Ghana, Leklebi-Agbesia , 6°56′ 1.3812″N, 0°29′1.5612″W, 19 July 2005, leg. A.C. Agyei & A. Hillers GoogleMaps ; ZMB 79187 View Materials (field #: GO14 ), adult male, Liberia, Gola National Forest , 7°27′10.6812″N, 10°41′31.3188″W, 28 November 2005, leg. A. Hillers GoogleMaps ; ZMB 79197 View Materials (field #: G84), adult female, Ghana, Volta Region, 22 August 2001 , leg. M.-O. R ̂del; ZMB 79210 View Materials (field #: GOL 060 , GenBank #: 16S: MG552310 ), adult male, Sierra Leone, Gola North Forest Reserve , 7°35′10.14″N, 11°1′30.9612″W, 15 September 2005, leg. A. Barrie & A. Hillers GoogleMaps ; ZMB 79253 View Materials (field #: GRE 010 , GenBank #: 16S: MG552307 ), adult female, Liberia, Grebo National Forest , 5°24′6.48″N, 7°44′0.6612″W, 7 December 2005, leg. A. Hillers GoogleMaps ; ZMB 87105–06 View Materials (field #: P.LI 12 004–P.LI 12 005, GenBank #: 16S: MG552296 & MG552305 ), adult female and male, Liberia, Gba Community Managed Forest , 7°28′52.6584″N, 8°34′28.5564″W, 28 January 2012, leg. J. Penner GoogleMaps ; ZMB 87107 View Materials (field #: P.LI 12 077, GenBank #: 16S: MG552312 ), adult male, Liberia, Gba Community Managed Forest , 7°30′34.5636″N, 8° 41′50.7408″W, 3 February 2012, leg. J. Penner GoogleMaps ; ZMB 87108 View Materials (field #: P.LI 12 321, GenBank #: 16S: MG552315 ), adult male, Liberia, Sapo National Park , 5°29′42.8352″N, 8°21′55.1988″W, 5 September 2012, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 87109 View Materials (field #: P.LI 12 466, GenBank #: 16S: MG552323 ), adult male, Liberia, Mount Ghi South , 5°38′34.9944″N, 8°12′30.1428″W, 29 September 2012, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 87110–11 View Materials & 87116 (field #: P.LI 12 503–P.LI 12 505, GenBank #: 16S: MG552319 & MG552320 ), adult male, female and male, Liberia, Mount Ghi South , 5°39′11.124″N, 8°12′21.708″W, 3 October 2012, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 87112 View Materials (field #: P.LI 12 300, GenBank #: 16S: MG552322 ), adult male, Liberia, Sapo National Park , 5°31′20.8272″N, 8°20′46.2084″W, 14 September 2012, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 87113 View Materials (field #: P.LI 12 377, GenBank #: 16S: MG552317 ), adult male, Liberia, Sapo National Park , 5°30′18.5652″N, 8°20′14.9208″W, 18 September 2012, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 87114 View Materials (field #: P.LI 12 432, GenBank #: 16S: MG552316 ), adult female, Liberia, Mount Swa , 6°26′18.0636″N, 9°3′25.9416″W, 24 September 2012, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 87115 View Materials (field #: P.LI 12 279, GenBank #: 16S: MG552314 ), adult female, Liberia, Sapo National Park , 5°31′34.8564″N, 8°20′53.1636″W, 12 September 2012, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 88438 View Materials (field #: RG07 ), adult female, Liberia, Krahn-Bassa Proposed Protected Area , 06°02′34.8″N, 08°20′10.3″W, 25 March 2018 GoogleMaps , leg. M.-O. R ̂del & J. Glos ; ZMB 88512 View Materials (field #: RG80 ), adult male, Liberia, Foya Proposed Protected Area , 08°00′21.3″N, 10°25′20.2″W, 5April 2018, leg. M.-O. Rödel & J. Glos GoogleMaps ; ZMB 88549 View Materials (field #: RG118 , GenBank #: 16S: OQ400915 ), adult male, Liberia, Foya Proposed Protected Area , 08°03′18.6″N, 10°23′04.0″W, 8 April 2018 GoogleMaps , leg. M.-O. R ̂del & J. Glos .
Additional material. Ten males, ten females; ZMB 90052 View Materials (field #: N035), adult female, Guinea, Keoulenta , 7°41′20.6”N, 8°21′19.0”W, 21 September 2009, leg. J. Doumbia, L. Sandberger, K. Camara & F. Gbêmou GoogleMaps ; ZMB 90053 View Materials (field #: N064), adult female, Guinea, Foromota , 7°42′58.3”N, 8°21′41.6”W, 3 November 2009, leg. J. Doumbia, L. Sandberger, K. Camara & F. Gbêmou GoogleMaps ; ZMB 90054 View Materials (field #: S4- Dian 91), adult male, Guinea, Diandian Missidé , 10°59′10.356”N, 13°47′44.556”W, 3 October 2016, leg. J. Doumbia & K. Camara GoogleMaps ; ZMB 90055 View Materials (field #: Ban _0362), adult female, Guinea, Banco , 8°31′6.312”N, 8°56′15.864”W, 17 May 2019, leg. J. Doumbia & K. Camara GoogleMaps ; ZMB 90056 View Materials (field #: G009), adult female, Liberia, Gangra , 7°33′47.556”N, 8°38′15.144”W, 14 January 2009, leg. J. Doumbia, K. Camara GoogleMaps & A. T. Johnson ; ZMB 90057 View Materials (field #: Wata 2_0201), adult female, Guinea, Wataférédou , 8°39′15.192”N, 8°51′11.196”W, 8 May 2019, leg. J. Doumbia & K. Camara GoogleMaps ; ZMB 90059 View Materials (field #: G003), subadult female, Liberia, Gangra , 7°32′40.992”N, 8°37′35.58”W, 12 January 2009, leg. J. Doumbia, K. Camara GoogleMaps & A. T. Johnson ; ZMB 90060 View Materials (field #: G009), adult male, Liberia, Gangra , 7°33′47.556”N, 8°38′15.144”W, 14 January 2009, leg. J. Doumbia, K. Camara GoogleMaps & A. T. Johnson ; ZMB 90062 View Materials (field #: Obay _0548), adult male, Liberia, Obayanmai , 8°8′59.82”N, 9°52′49.764”W, 7 April 2019, leg. J. Doumbia, K. Camara, F. Gbêmou & F. B. Beyan GoogleMaps ; ZMB 90063 View Materials (field #: Obay _0611), adult male, Liberia, Obayanmai , 8°8′56.04”N, 9°52′26.436”W, 9 April 2019, leg. J. Doumbia, K. Camara, F. Gbêmou & F.B. Beyan GoogleMaps ; ZMB 90159 View Materials (field #: PG.L.15/040), adult female, near Bouaflé , Ivory Coast, 7°0′15.1488”N, 5°34′37.0344”W, 7 May 2015, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 90161 View Materials (field #: PG.L.13 033), subadult female, Liberia, Dugbe , 5°5′15”N, 8°36′29.88”W, 26 August 2013, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 90162 View Materials (field #: PG.L.13 109), adult male, Liberia, Dugbe , 5°8′40.92”N, 8°29′57.12”W, 3 September 2013, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 90163 View Materials (field #: LI10 014), adult male, Liberia, Tokadeh , 7°26′40.704”N, 8°39′27.972”W, 11 November 2010, leg. J. Penner GoogleMaps ; ZMB 90164 View Materials (field #: PG.L.13 054), adult female, Liberia, Dugbe , 5°4′20.28”N, 8°33′23.76”W, 28 August 2013, leg. J. Penner & N.L. Gonwouo GoogleMaps ; ZMB 90165 View Materials (field #: LI10 121), adult male, Liberia Tokadeh , 7°27′52.164”N, 8°39′53.928”W, 27 November 2010, leg. J. Penner GoogleMaps ; ZMB 90166 View Materials (field #: LI10 114), adult male, Liberia, Eastern Nimba Nature Reserve , 7°29′3.264”N, 8°34′36.264”W, 26 November 2010, leg. J. Penner GoogleMaps ; ZMB 90167 View Materials (field #: LI10 136), adult male, Liberia, Geipa , 7°28′59.196”N, 8°32′13.344”W, 3 December 2010, leg. J. Penner GoogleMaps ; ZMB 90168 View Materials (field #: PG.L.13 188, adult male, Liberia, Dugbe, 5°5′45”N, 8°29′32.64”W, 13 September 2013, leg. J. Penner & N.L. Gonwouo; GoogleMaps ZMB 90169 View Materials (field #: LI10 120, adult female, Liberia, Eastern Nimba Nature Reserve , 7°29′3.264”N, 8°34′36.264”W, 28 November 2010, leg. J. Penner. GoogleMaps
Diagnosis. Amnirana parva sp. nov. differs from other West African Amnirana genetically, morphologically, and acoustically. The uncorrected pairwise-divergence in 16S ribosomal RNA between the new species and its closest congener, A. fonensis , is 5.8%. The new species can be distinguished from A. fonensis by several morphological traits in male specimens. SVL of male A. parva sp. nov. ( 41.9–50.4 mm) is smaller than in A. fonensis ( 48.7–65.3 mm). Area of humeral gland is smaller in A. parva sp. nov. ( 3.7–14.8 mm 2) than in A. fonensis ( 13.5–26.4 mm 2) as well. Males of A. occidentalis and A. galamensis reach larger SVL (67 and up to 80 mm, respectively; females of these species reach 96 and 86 mm, respectively), have differing color pattern and completely smooth dorsal skin (R̂del & Bangoura 2004; Channing & R̂del 2019). Most males of A. parva sp. nov. have dark spots on the dorsum. Breeding males of A. fonensis differ by a yellow dorsal coloration. The ranges of two Central African Amnirana species that are morphologically similar to A. parva sp. nov., A. albolabris (comprising various lineages, see Jongsma 2018) and A. asperrima ( Perret, 1977) , do not overlap with the new species. Nominotypical A. albolabris from Central Africa show a larger body size in males ( Perret 1977 reports 44–57 mm SVL for A. albolabris from Cameroon). Amnirana asperrima can also be distinguished by both its larger adult body size (SVL of males: 46.7–60.1 mm) and the texture of its dorsal skin, which is densely covered with tiny spines. Other Central African Amnirana species show distinct morphological traits and cannot be confused with Amnirana parva sp. nov.: A. amnicola ( Perret, 1977) adults are larger (SVL of males and females: 53–72 mm) and have a more pointed snout; and A. lepus (Andersson, 1903) adults are much larger (SVL: 70–100 mm), have finely granular skin, fully webbed toes, and much longer hind limbs. Last, the new species also differs from populations previously referred to A. longipes (Perret, 1960) that are much larger (SVL 77–87 mm) and have a more robust body and longer hind limbs. Although we follow Jongsma (2018) in recognizing A. longipes as a junior synonym of A. albolabris , we include it here in anticipation that this may be recognized as a valid taxon if one or several lineages of Central African A. albolabris are recognized as distinct species.
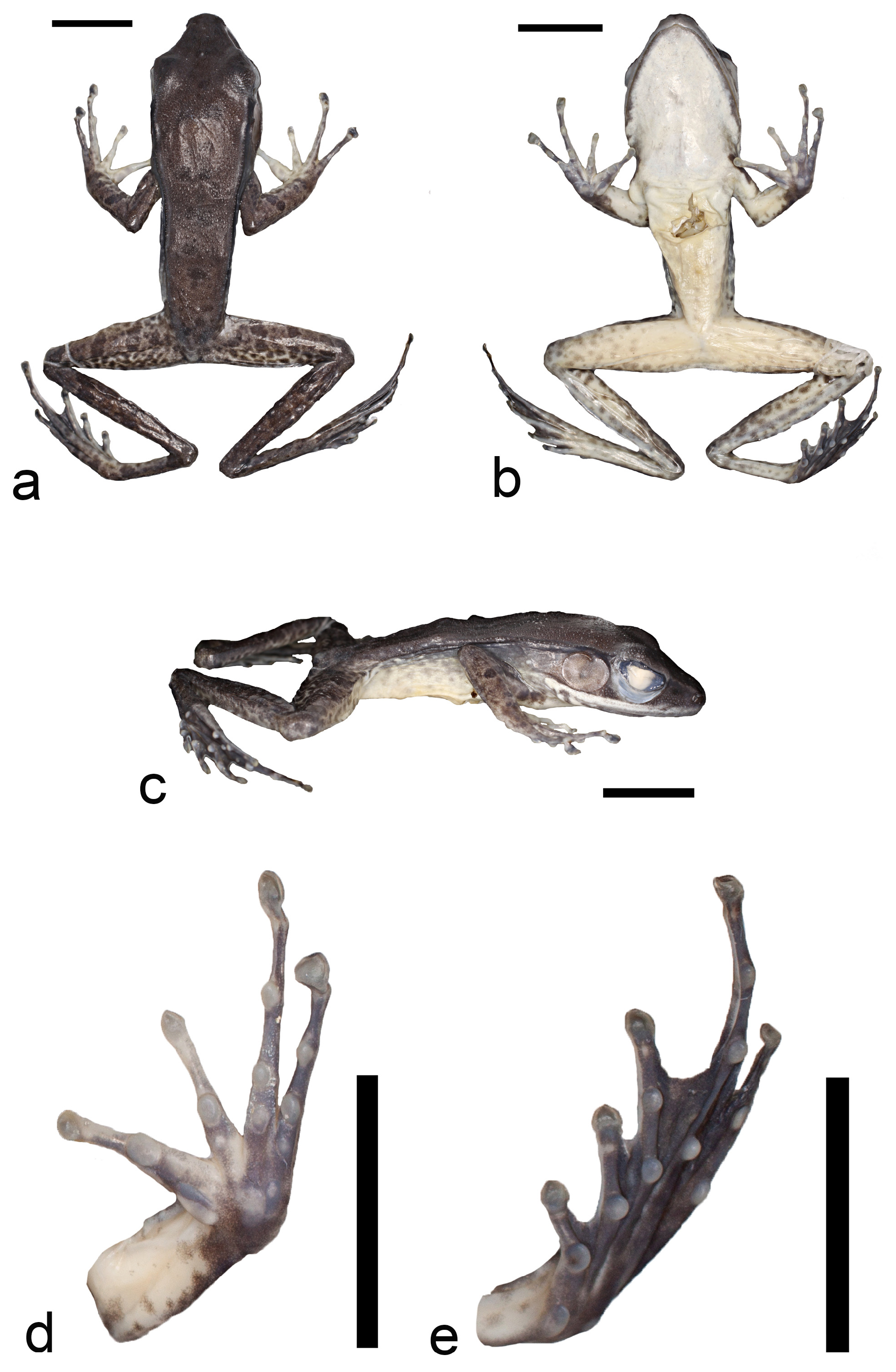
Description of the holotype. Figs. 5–6 View FIGURE 5 View FIGURE 6 ; measurements in mm. Adult male with long and slender body, slightly dorsoventrally flattened; snout–vent length 42.9; snout acuminate with rounded tip in dorsal view, narrowly rounded in lateral view; head length 15.8, approximately 27% of SVL; head width 13.6; eye–snout distance 6.7; nostrils directed laterally; nostril closer to the snout (1.7) than to eye (4.7); eye diameter 6.1, slightly larger than tympanum diameter (5.2); interorbital distance 4.3; approximately one third of eye projecting above dorsal margin of head in lateral view; canthus rostralis distinct and sharply protruding; loreal region concave; anterocranial part of upper arm with large protruding gland (3.6 long, 2.0 wide, surface: 7.2 mm 2); long and slender fingers, tips broader than subarticular tubercles, forming discs, reaching 130–200% of finger width, inner metacarapal tubercle large and elongated, outer metacarpal tubercle smaller and more rounded; manual webbing absent; finger formula II<I<III<IV; nuptial pads on anteroventral surface of first finger, almost transparent; thigh length 20.9, approximately half SVL; crus length 23.0; foot length including longest toe 32.5; single inner metatarsal tubercle large and elongated; single outer metatarsal tubercle small and round; toes long and slender, tips slightly broader than subarticular tubercles, forming small discs; toe formula I<II<V<III<IV; webbing formula I(0) II(1-0) III(1-0) IV(2-1) V(0); head and dorsum covered with small tubercles, becoming slightly larger from snout to middle of dorsum, decreasing in size towards lower back; narrow, distinct dorsolateral ridge extending from posterior corner of eye, reaching to posterior 1/3 of body, in last third broken into isolated small bumps; ventral skin smooth.
Dorsal coloration in life ( Fig. 6a View FIGURE 6 ) medium brown with few darker spots; flanks greenish yellow, becoming paler ventrally with some darker mottling; arms slightly lighter brown than dorsum, marbled with fine, irregular, dark pattern; hind limbs slightly lighter brown than dorsum with narrow transverse dark bars; posterior parts of thighs marbled; foot webbing greyish brown; throat and belly white; ventral side of legs pale beige; hind limbs ventrally patterned with indistinct brown spots; bright, white upper lip extending posterior to angle of mouth, below and posterior to tympanum. After four years in preservation (75% ethanol; Fig. 5 View FIGURE 5 ), in general less saturated colors: brownish dorsal color darkened; greater contrast of marbled limb patterning; flanks lacking yellow coloration, now beige with small remnants of brownish speckles; white throat and lip more shiny.
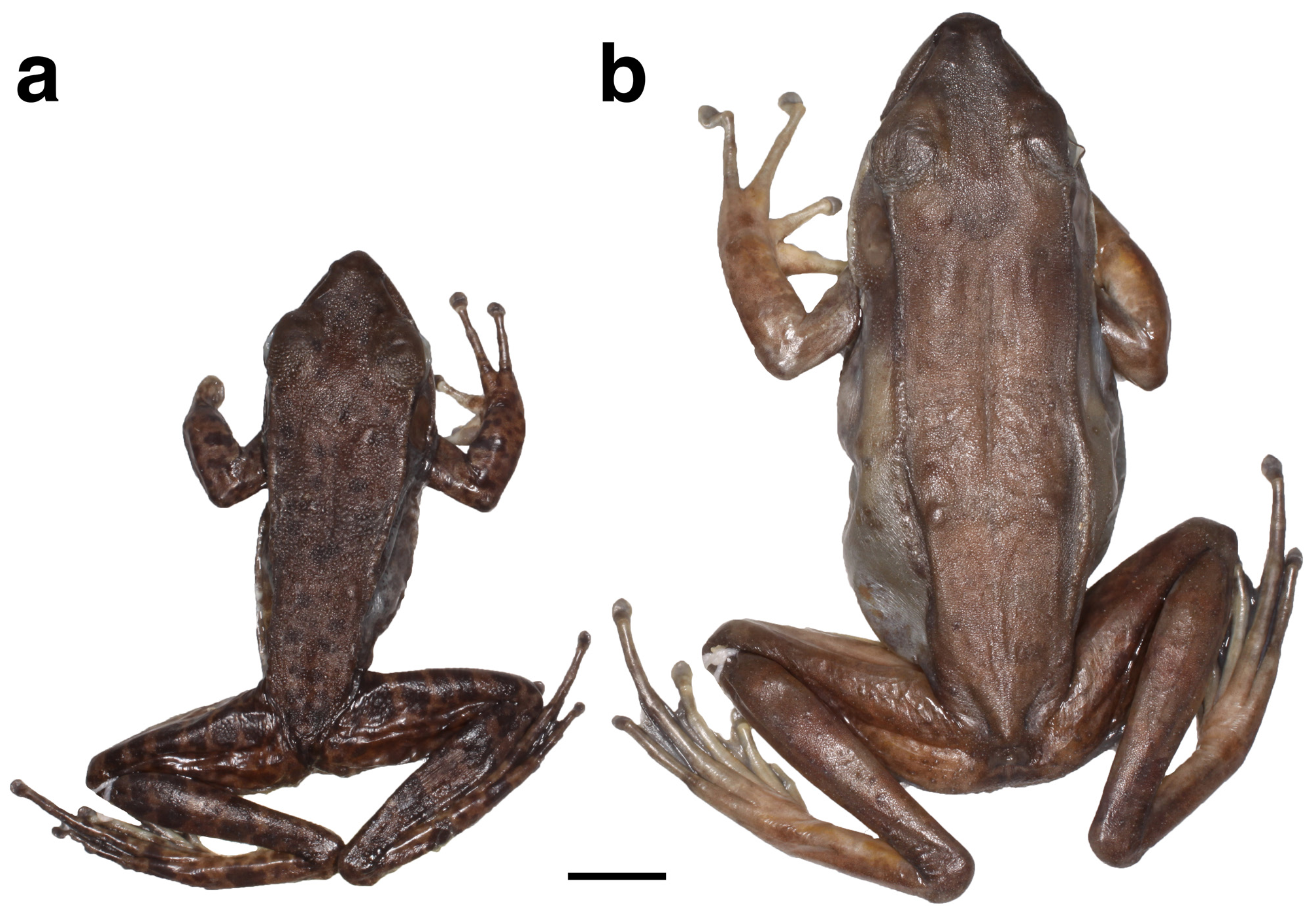
Variation. Measurements (in mm) of all individuals are summarized in Table 5. Overall, the male paratypes were similar to the holotype in external appearance and coloration. Male SVL ranged from 41.9–50.4 (mean ± sd: 45.9 ± 2.9, N= 13). The humeral gland area spanned between 3.7–14.8 mm 2 (9.4 ± 2.7 mm 2, N= 13). Female SVL ranged from 53.6–75.6 (63.9 ± 7.8, N= 10) thus markedly exceeding male SVL ( Fig. 7 View FIGURE 7 ). Seven of 13 showed slightly more extended (1-1), and a single male paratype showed slightly less extended webbing (2-2) of the fourth toe. In female paratypes seven of 10 individuals exhibited a webbing formula with slightly more extended webbing on toe IV(1-1). The dorsal skin was covered with small tubercles in all individuals, but females had fewer, smaller, and less spiny tubercles. In some individuals, the skin appeared almost smooth at first sight, but tiny granules were always present upon detailed inspection. The dorsolateral ridge was interrupted in the majority of specimens: females (six of 10) and males (nine of 13).
Females showed a brighter brown dorsum and few to no dark spots (see Figs. 6b View FIGURE 6 , 7b View FIGURE 7 , 8e, g View FIGURE 8 , 11c View FIGURE 11 ). On the dorsum of males, the ground color varied from chocolate-brown to dark brown and all males had at least some dark spots. The ventral coloration varied greatly among males. Among the paratypes, some had a plain white throat (e.g., ZMB 88549, 87112), similar to the holotype, whereas others exhibited dusty brown speckling covering almost the entire throat (e.g., ZMB 87113). The belly of all males were white to cream, and some individuals had irregular brown spots (e.g., ZMB 87110) that were densest near the legs, throat, or flanks, but were absent at the center of the venter. Females showed some color variation on the venter. In ZMB 87114, the belly and throat were white, whereas ZMB 79253 and ZMB 79197 had a cream-colored belly and a dusty brown speckled throat.
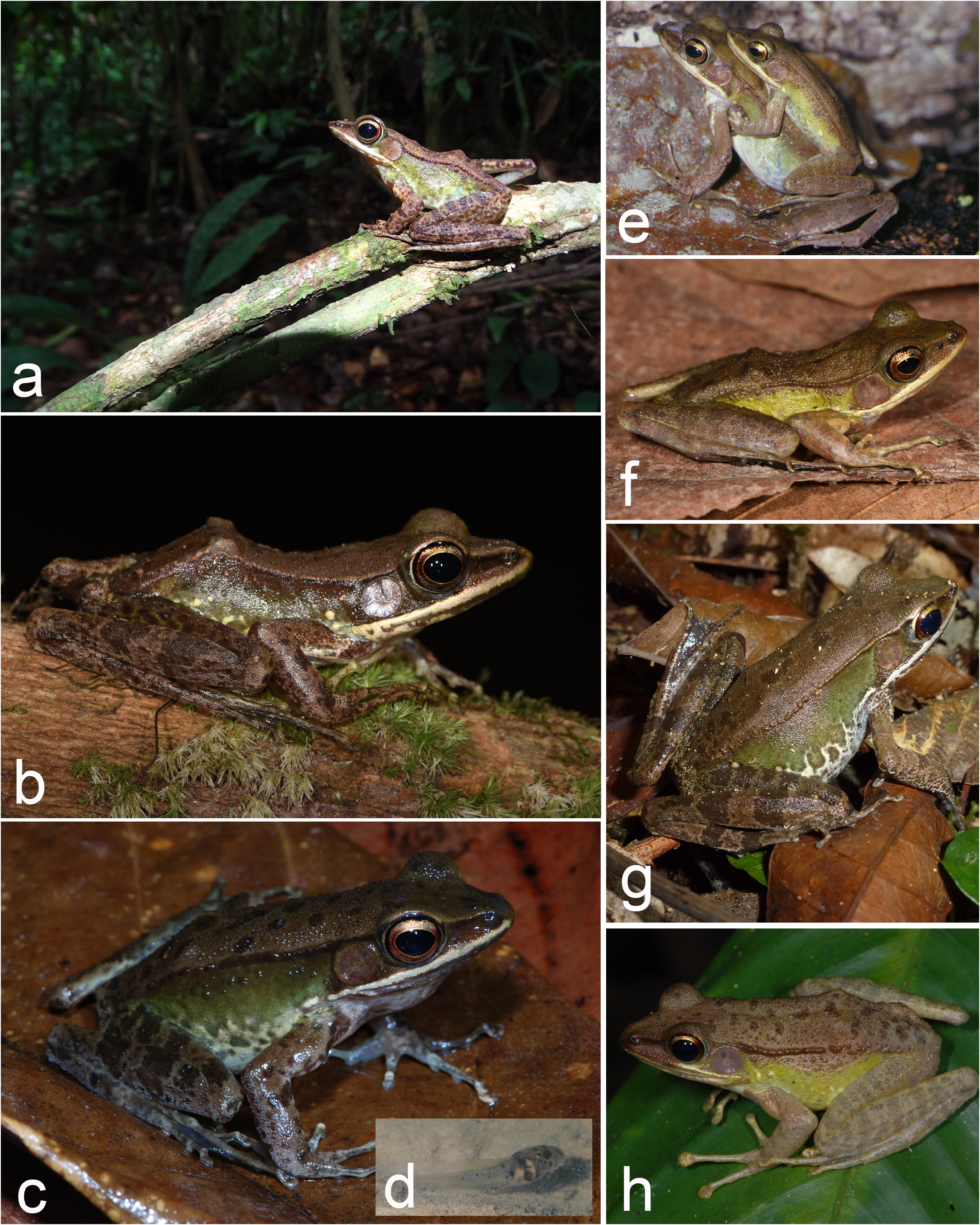
Concerning life coloration ( Figs. 8 View FIGURE 8 , 11 View FIGURE 11 ), the dorsum exhibited various shades of brown, sometimes with darker spots (especially in males, most females without darker spots). The flanks in males were greenish yellow ( Fig. 8f, h View FIGURE 8 ) to chocolate brown dorsally ( Fig. 8b View FIGURE 8 ), usually turning lighter ventrally (but see Fig. 8a View FIGURE 8 ). The flanks in females were olive green, turning paler ventrally ( Figs. 8c, e, g View FIGURE 8 , 11c View FIGURE 11 ), and becoming almost cream-colored towards the belly (then often with some greenish or brownish mottling). The tympanum was brown in both sexes. Males had a narrow green line surrounding the eyes. In both sexes the iris was golden, sometimes with a reddish tinge, with the lower half of the iris being darker. The limbs and dorsum were a similar shade of brown in both sexes. The limbs sometimes were somewhat lighter, but with darker patterning. The number of conspicuous dark transverse bars on the hind limbs varied among individuals (e.g. compare Figs. 8b & 8h View FIGURE 8 ). The venter was beige or cream. Both sexes had a light cream or white upper lip, and rarely the anterior part of the lip was brownish ( Fig. 8h View FIGURE 8 ).
Juveniles showed a more contrasting pattern compared to adults. It consisted of a greenish brown dorsum with black spots and an almost black loreal region and flank. The ventral part of the flank and the belly were white with black dots. The juveniles had more conspicuous dark cross bands on the hind limbs and blackish dots on the forelegs; a bright white upper lip, and a bright red iris (uppermost part golden) ( Fig. 11f View FIGURE 11 ).
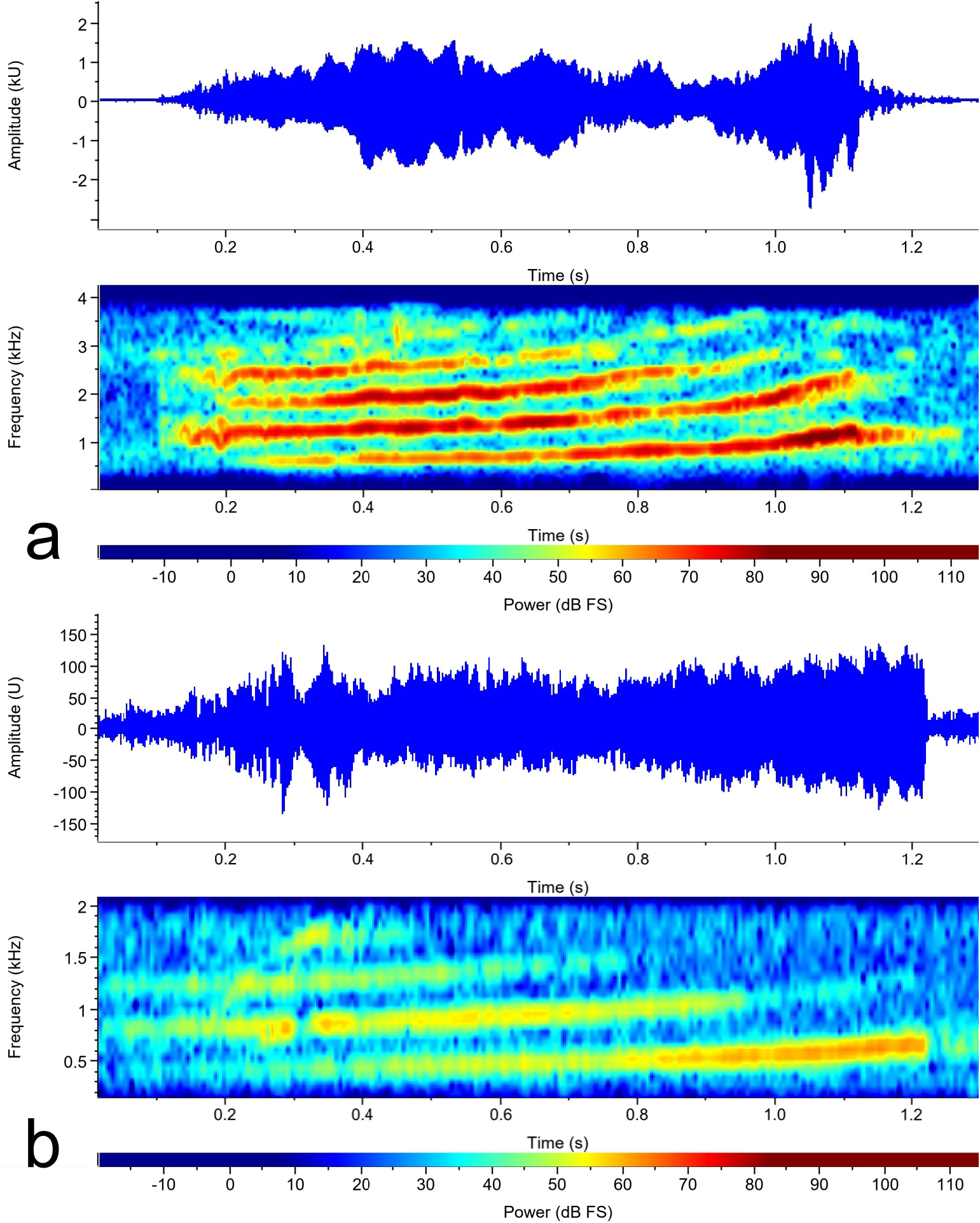
Acoustics. The advertisement call of Amnirana parva sp. nov. consists of a whiny-voiced croaking sound with ascending frequency modulation from 932 to 1092 Hz (mean ± sd: 1030 ± 86, N= 3). The sound consists of up to five harmonics of which the second-lowest is the dominant one in the first half, the lowest one in the second half ( Fig. 4a View FIGURE 4 ). The call duration ranges from 0.26– 0.78 s (0.52 ± 0.26, N= 3). Dominant frequency at the beginning of the call ranges from 948–1034 Hz (1004 ± 49, N= 3). All call characteristics are summarized in Table 4. A View TABLE 4 call description by Schiøtz (1964c) from central Sierra Leone seems to refer to our new species. In that paper, Schiøtz describes three different sections of the call (such a clear distinction of sections was not detected by us). Call duration and complexity, however, seem to increase with the motivation of the frogs (MOR pers. obs.). Schiøtz (1964c) also mentions that these calls were different from those he recorded in Cameroon for A. albolabris , though he did not provide further details.
Distribution. Confirmed A. parva sp. nov. records so far are known from western Sierra Leone to southerncentral Ghana, all within the belt of the (former) rainforest zone, bordering the southern humid Guinean savanna ( Fig. 9 View FIGURE 9 ). So far, no confirmed genetic records exist for Ivory Coast. However, morphologically we could assign frogs from the Taï National Park, the Azagny National Park, the Banco National Park, the Haute Dodo, Cavally and Tanoé forest reserves, as well as the Ivorian parts of Mount Nimba to the new species. Other formerly published records of A. albolabris (see literature cited below) from this country from habitats that include primary lowland evergreen and humid but semi-deciduous forest cannot be unambiguously assigned to either A parva sp. nov. or A. fonensis (see below).
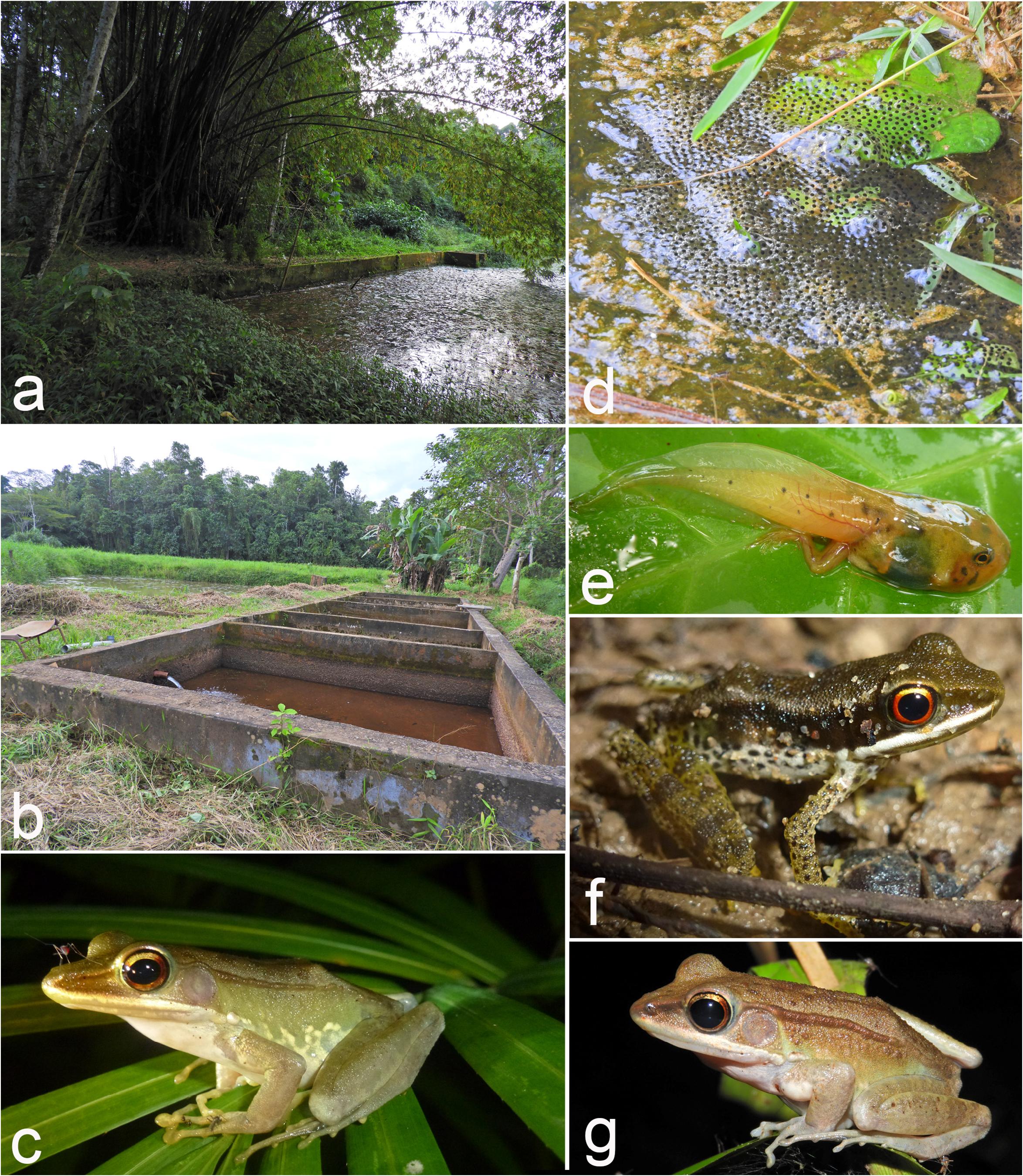
Life history. It is difficult in many cases to assign previous field observations and literature records to A. parva sp. nov. or A. fonensis . We summarize observations based on confirmed records (morphology and genetics of vouchers, and/or acoustics) as well as records, which originate from primary humid-evergreen rainforest. The latter decision was based on the results of the environmental niche modelling (see below). All observed and collected individuals of A. parva sp. nov. were encountered in closed primary or slightly degraded lowland rainforest, on the banks of or near to small to medium-sized streams ( Fig. 10 View FIGURE 10 ). In Banco National Park, southeastern Ivory Coast, the species was recorded in the forest, forest patches dominated by bamboos, and in man-made ponds of a fish-farm in a forest clearing ( Fig. 11a, b View FIGURE 11 ). Although we often heard small choruses (< 10 males), we only rarely found clutches of eggs. Clutches comprised more than 1000 eggs (compare Perret 1977 for data on Cameroonian populations of A. albolabris ), that were floating on the water surface. The clutches are often attached to dead branches in the water. In Banco National Park, females attached their eggs to floating vegetation ( Nymphaea sp. ), or grasses growing in the water. The eggs of one clutch were counted ( Fig. 11d View FIGURE 11 ) and contained 3,072 eggs. The dark brown and white eggs had a diameter ranging from 1.3 to 2.4 mm (mean ± sd: 2.1 ± 0.1 mm, N= 100). These eggs, although deposited in the fish-breeding ponds, were not preyed upon by Coptodon fish ( Cichlidae ). Juveniles sometimes have been seen further away from water, in the forest leaf-litter. They were particularly abundant in bamboo patches. The species seems to be mainly diurnal, with both adults and juveniles being active on or only slightly off the ground during daytime. At night, adults were frequently observed sitting higher up (sometimes as high as 2–3 m) on branches of shrubs and trees and on palm leaves ( Figs. 8a View FIGURE 8 , 11c, g View FIGURE 11 ). However, daily activity may differ between differently closed forests. Diurnal activity was observed in closed canopy forest, such as Taï National Park, Ivory Coast, or Foya Forest Reserve, Liberia. By contrast, in the more open Azagny National Park, the Mabi-Yaya Forest Reserve, and some village forests (i.e. Tanoé forests, Tiassalé, Yakassé-Mé, Daloa; all Ivory Coast), the frogs were abundant at night, but only rarely seen during the day. In Banco National Park, they were absent in the clearing during daytime, but at night they congregated around the fish ponds. Generally, we encountered call aggregations during day and night. Calling males sit exposed (but often still not easy to detect) and widely spaced on banks or on branches around deeper, almost stagnant parts of streams and ponds. Very often these sites are close to holes in the river/pond bank that are inhabited by a single Dwarf Crocodile ( Osteolaemus tetraspis ) or a family group of that species (MOR, unpubl. data from Taï National Park, Ivory Coast). It seems that the frogs are left unharmed by the crocodiles, most likely due to their toxic skin secretions. In two cases, one of us (MOR) touched his eyes after handling a frog, and although intensively washed with clean water—it kept burning for several hours.
The tadpole of A. albolabris sensu lato has been described and illustrated by Lamotte (1957), Guibé & Lamotte (1958), Perret (1977), and Channing (2012). These robust and conspicuously colored tadpoles (with red and black markings) ( Figs. 8d View FIGURE 8 , 11e View FIGURE 11 ) may reach large sizes (up to 60 mm) and can be observed actively foraging during daytime. In their habitat they are the only tadpoles observed swimming during the day. They have several large skin glands, which likely produce toxic substances ( Perret 1977) that make them unpalatable to fish and Xenopus , but apparently not to invertebrate predators such as spiders that hunt surfacing tadpoles ( McIntyre 1999). In Taï National Park, we offered A. cf. parva tadpoles to Clarias catfish (MOR, unpubl. data). Some fish took tadpoles into their mouth, but then immediately spat them out. Similar aposematically colored West African tadpoles have been reported from Phrynobatrachus maculiventris Guibé & Lamotte, 1958 (R̂del 2009).
The published descriptions of tadpoles of A. albolabris sensu lato are similar to one another, though definitely refer to multiple distinct taxa. The most detailed description, predominantly based on tadpoles from Mount Nimba in West Africa, was published by Lamotte (1957). These authors report on morphological differences between tadpoles from different West and Central African regions (including differences in color pattern and formulae of the keratodonts). They noted that tadpoles from the Simandou Range (the type locality of A. fonensis , see below) differ from those of Mount Nimba and are morphologically more similar to tadpoles from Central Africa. These differences were mainly having larger black dots on the tail, no spots on the throat, and the absence of a black axial line on the tail of tadpoles from Simandou. Future research should determine whether these differences indeed are species-specific.
IUCN threat status. Relatively little is known about the distribution and population sizes of A. parva sp. nov. Because available data indicate that it is broadly distributed in West African rainforests, it should probably be classified as ‘Least Concern’. This is based on its likely large range, extending from central Ghana and across the forest zone into eastern Sierra Leone (Extent of Occurrence = 224,764 km ²; Bachman 2011), as well as its tolerance to minor forest degradation.
Etymology. The specific epithet was chosen because it is the smallest member in the genus Amnirana . The Latin word for small, ‘parvus’, has been adjusted to the female ‘ Amnirana’.
| MOR |
Museum of the Rockies |
| R |
Departamento de Geologia, Universidad de Chile |
| T |
Tavera, Department of Geology and Geophysics |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.