Microstomum rubromaculatum von Graff, 1882
|
publication ID |
https://doi.org/ 10.5852/ejt.2018.398 |
|
publication LSID |
lsid:zoobank.org:pub:58C075B0-7409-41B7-A6F4-900A5A6BFECE |
|
DOI |
https://doi.org/10.5281/zenodo.5991931 |
|
persistent identifier |
https://treatment.plazi.org/id/BD084F11-FFC0-6D1B-07E4-FD2BFDB3F8CC |
|
treatment provided by |
Plazi |
|
scientific name |
Microstomum rubromaculatum von Graff, 1882 |
| status |
|
Microstomum rubromaculatum von Graff, 1882
Figs 4–5 View Fig. 4 View Fig. 5
Material examined
SWEDEN: 20 live specimens, Fiskebäckskil, Kristineberg Sven Lovén Center for Marine Research , 58°14′59″ N, 11°26′45″ E, 20 Aug. 2015, marine, sublittoral phytal on algae, M. Curini-Galletti leg. (Genbank accession MF185684 View Materials -96). GoogleMaps
Type locality
ITALY: Gulf of Naples, Tyrrhenian Sea. Deposition not recorded.
Habitat
Marine, sublittoral phytal in algae (e.g., Sargassum sp.) or benthal on shells, fine sand and mud.
Distribution
Ireland: New Harbor , Galway, 1–2 m; Malahide Inlet , Dublin, 2 m ( Southern 1936).
England: Wembury ( Meixner 1938).
Faroe Islands: Vaagfjord , Suderø, 10 m ( Steinböck 1931).
France: Concarneau (von Graff 1913).
Iceland: North of Ísafjörður , 1–2 m ( Steinböck 1938).
Sweden: Gullmar Fjord, Fiskebäckskil, 1–2 m ( Westblad 1953; pers. obs. by author).
Norway: Herdla (Westblad 1934; Karling 1953).
Population description
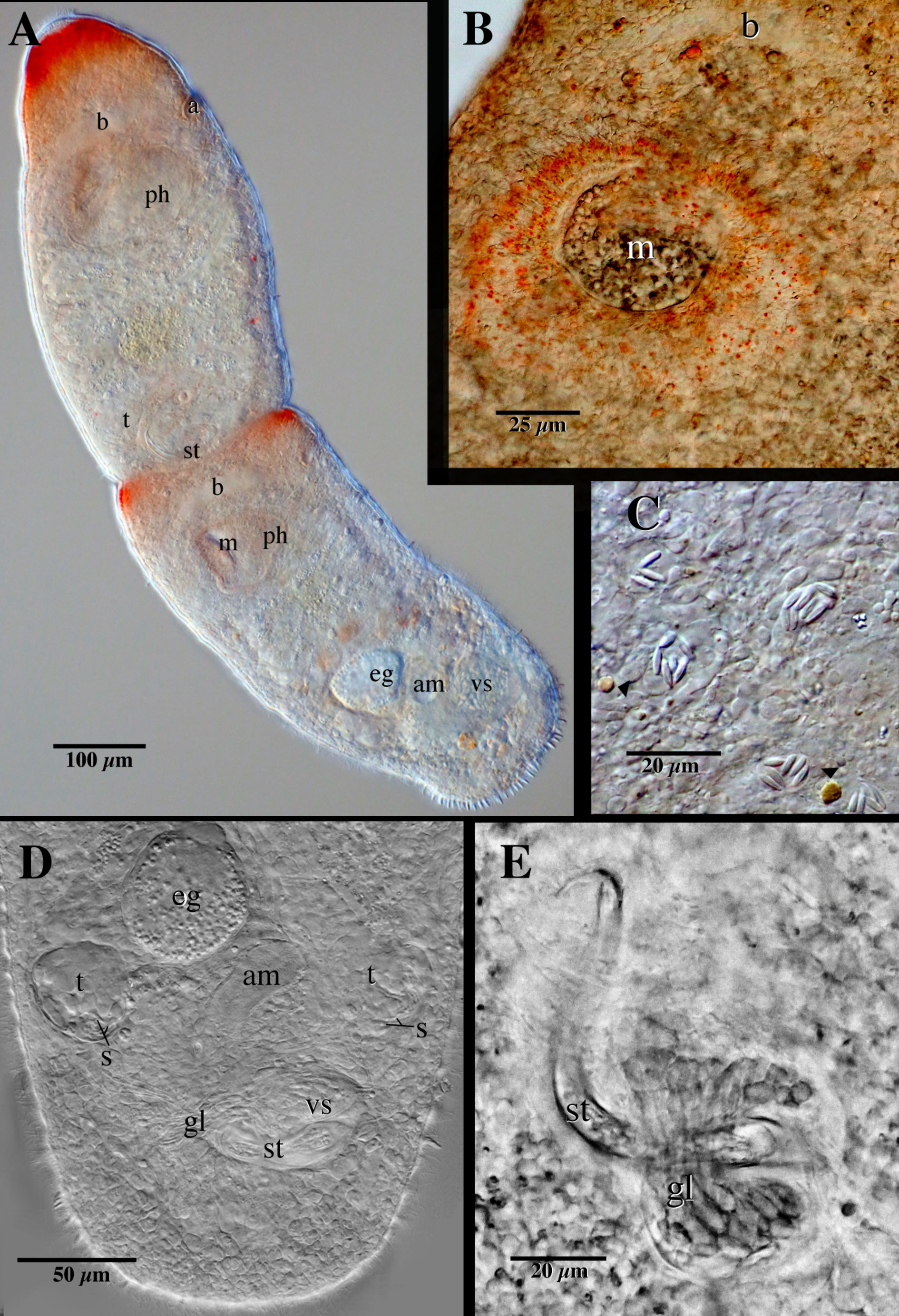
Microstomum with field of bright red pigmentation spots on each well-developed zooid; length of pigmentation stretches from just below the anterior tip to halfway to the brain, width of pigmentation somewhat variable: either predominately at the lateral margins and thinning toward the middle or, most frequently, a band that encircles the entire body ( Fig. 4A View Fig. 4 ). Other small orange-red droplets may be scattered within the parenchyma of some specimens, particularly around the anterior and pharynx ( Fig. 4B–C View Fig. 4 ). Body otherwise colorless, clear and reflective of intestine.
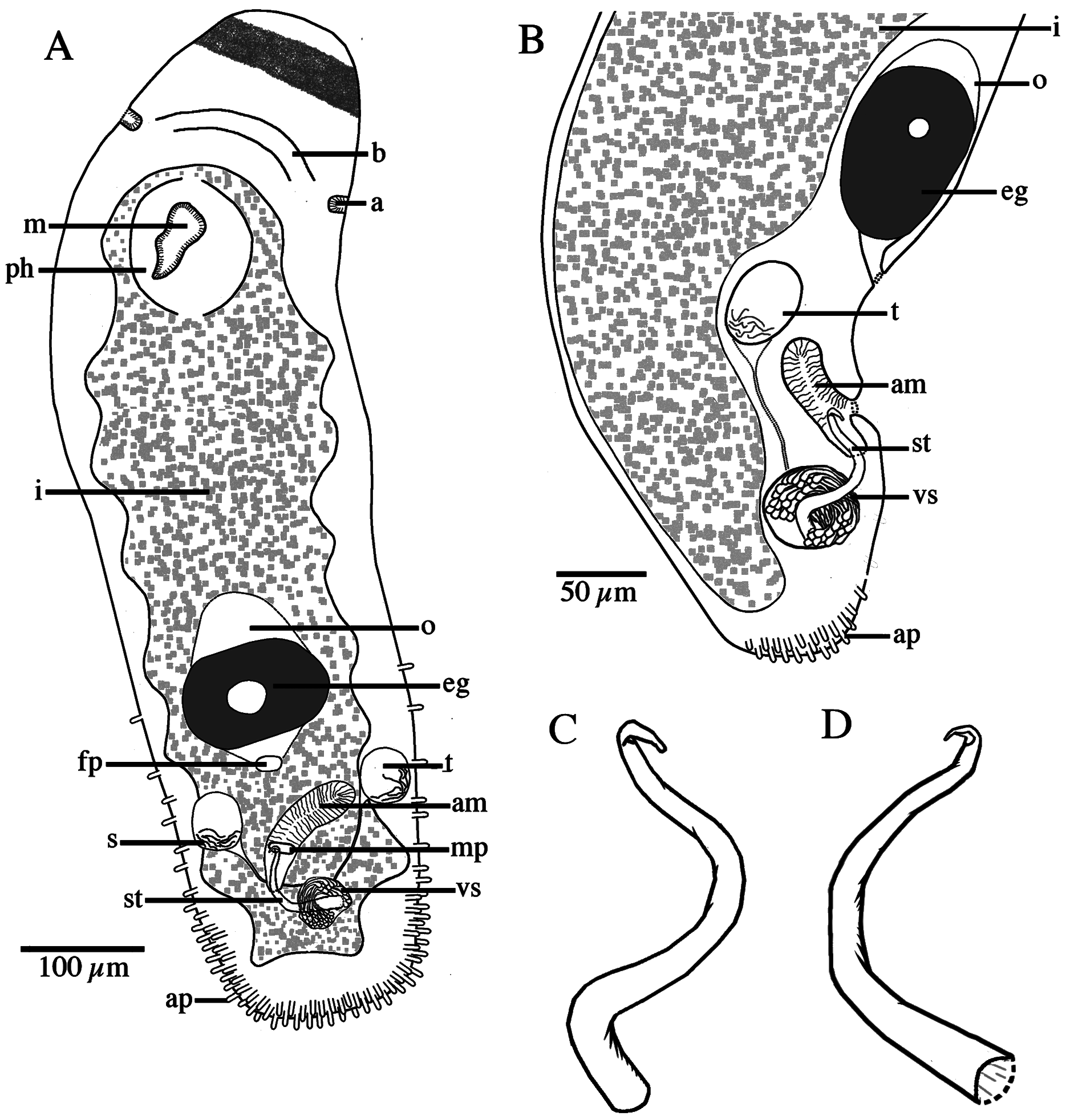
Vegetative chains to four zooids long; maximum animal/zooid body length 2000/1400 µm. Body width generally consistent, accepting slight constrictions between zooids and at the level of the ciliary pits; slightly tapering toward the rounded anterior end. Posterior end bluntly rounded. Posterior rims of welldeveloped zooids with many 5–6-µm-long posterior adhesive papillae ( Fig. 5 View Fig. 5 ).
Epidermis uniformly covered with cilia. Bundles of 3–8 nematocysts present, scattered in the parenchyma ( Karling 1966); each nematocyst 4–6 µm long ( Fig. 4C View Fig. 4 ). According to Westblad (1953), expelled nematocysts along the lateral body margins may resemble papillae.
Mouth slit-like at rest, but able to distend to encompass very large food. Pharynx spherical to elliptical, encompassing up to the length of the second quarter of the zooid. Preoral gut extending to brain or slightly anterior. Intestine yellow-brown or tinged with red or pink; may contain ingested prey.
Male reproductive system with paired testes located anterolaterally to male copulatory apparatus and gonopore ( Figs 4D View Fig. 4 , 5 View Fig. 5 ). Testes round, average diameter 47 µm, containing little or no sperm. Vasa defferentia connect individually to circular vesicula seminalis. Numerous prostatic glands insert anteriorly in vesicula seminalis and extend ventrally around the center of stylet ( Fig. 4D–E View Fig. 4 ). Stylet a single, wide spiral bent around a 90° angle, terminating in a ~10-µm-long fingerlike hook ( Figs 4E View Fig. 4 , 5C–D View Fig. 5 ); average length 95 µm (range 75–112 µm); width largest at the base, ~12 µm, tapering only slightly towards the distal end, ~5 µm at the base of the hook; opening subterminal. Stylet projects into a ciliated antrum masculinum ( Fig. 4A, D View Fig. 4 ).
Female reproductive system typical for the genus ( Figs 4D View Fig. 4 , 5 View Fig. 5 ). Single ovary situated mid-body, ventral to intestine, leading to ciliated female antrum. Female gonopore separate. Eggs develop caudally.
Remarks
Collected specimens generally appeared morphologically similar to the type description (von Graff 1882) and to previous accounts of M. rubromaculatum from Fiskebäckskil. Westblad (1953) recorded M. rubromaculatum from Fiskebäckskil with vegetative chains up to four zooids long, yet all currently collected specimens except one were composed of either two weakly developed zooids separated by a faint fission plane or one or two well-developed zooids only. This follows other patterns found in species of Microstomum in which slender chains of multiple, short zooids dominate during the asexual reproductive phase of the lifecycle while larger single or double zooid animals dominate during periods of sexual reproduction ( Bauchhenss 1971).
The amount of eyespot pigmentation in M. rubromaculatum can greatly vary between individuals. Specimens from Fiskebäckskil generally agreed with the original description of Graff (1882): paired, lateral eyespots composed of an accumulation of red pigmentation that extends medially to form a ring around the anterior end. However, pigmentation spots in four of the observed specimens remained clearly distinct, a phenomenon that has been recorded in other populations of M. rubromaculatum (von Graff 1913; Steinböck 1931). COI sequences were identical between specimens with two distinct eyespots and those with a circular band, which indicates amount of pigmentation is not necessarily a systematically important character. Rather, accumulation of eyespot pigmentation may be more “correlated with light intensity”, as in, e.g., Microstomum lineare ( Bauchhenss 1971) . Steinböck (1938) reported a single specimen of M. rubromaculatum from Iceland with a large central pigment spot that thinned toward the body margins. However, such a pattern was not observed in any of our specimens, nor otherwise recorded in any other population.
Red-orange droplets ( Fig. 4B–C View Fig. 4 ), that have not been previously documented in M. rubromaculatum , were observed in 18 of the 20 live specimens. The droplets ranged in size from a diameter of ~2–10 µm and were most often located anteriorly, especially around the pharynx. The droplets were most likely lipid deposits whose presence and coloration stems from ingested food. While such deposits have not been recorded before in Microstomum , colored lipid droplets are known to occur in other species of Macrostomorpha ( Rieger et al. 1991).
The distribution of Microstomum rubromaculatum is wide, with populations reported from the Mediterranean, the North Sea and the Baltic. Although such patterns do occur for other macrostomorphs (e.g., Macrostomum pusillum , M. rubrocinctum, Paramalostomum dubium – see Ax 1956; Karling 1974; Armonies 1988), including other species of Microstomum (e.g., M. lineare , M. papillosum – see Steinböck 1931; Karling 1974), the distribution may still be considered surprising giving the large geographic distances and differences in salinity and temperature ( Boyer & Levitus 1994). Evidence has increasingly shown that widespread taxa previously thought to represent a single species are in fact morphologically indistinct complexes. However, all our specimens were collected from a single location in west Sweden, and thus different populations of M. rubromaculatum could not be compared at this time. Sexually mature specimens of M. rubromaculatum have not been recorded from any other populations, including those inhabiting the type locality, and therefore further research may be necessary to confirm the identity of M. rubromaculatum from Fiskebäckskil, Sweden before sexual anatomy can be included in the description of the species as a whole.
Phylogeny
The maximum likelihood analysis found four moderately or highly supported clades of Microstomum ( Fig. 6 View Fig. 6 ). M. rubromaculatum was sister to M. edmondi sp. nov. with moderate support and further formed a clade with an unidentified species of Microstomum (species “D” in Janssen et al. 2015; see Table 1) and M. laurae sp. nov. The other three species of Microstomum represented in the analysis (species “B” in Janssen et al. 2015, M. lineare , M. papillosum ) individually comprised the remaining three clades. Patristic distances are presented in Table 2.
A true understanding of the evolutionary relationships within Microstomum would require multiple nuclear and mitochondrial gene sequences as well as a much greater species representation ( Maddison 1997). However, the results of the ML analysis and patristic distances presented here clearly separate specimens of M. edmondi sp. nov., M. laurae sp. nov. and M. rubromaculatum into three distinct lineages representing the three species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SuperOrder |
Macrostomorpha |
|
Order |
|
|
Family |
|
|
Genus |