Fergusoninidae Tonnoir, 1937
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4735.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:BD52DF91-3A7E-46FB-8975-38A67BFBBD61 |
|
DOI |
https://doi.org/10.5281/zenodo.3679568 |
|
persistent identifier |
https://treatment.plazi.org/id/BD15296C-6A5F-FFA0-FF1A-FB04DC30A7F2 |
|
treatment provided by |
Plazi |
|
scientific name |
Fergusoninidae Tonnoir, 1937 |
| status |
|
Fergusoninidae Tonnoir, 1937 View in CoL View at ENA
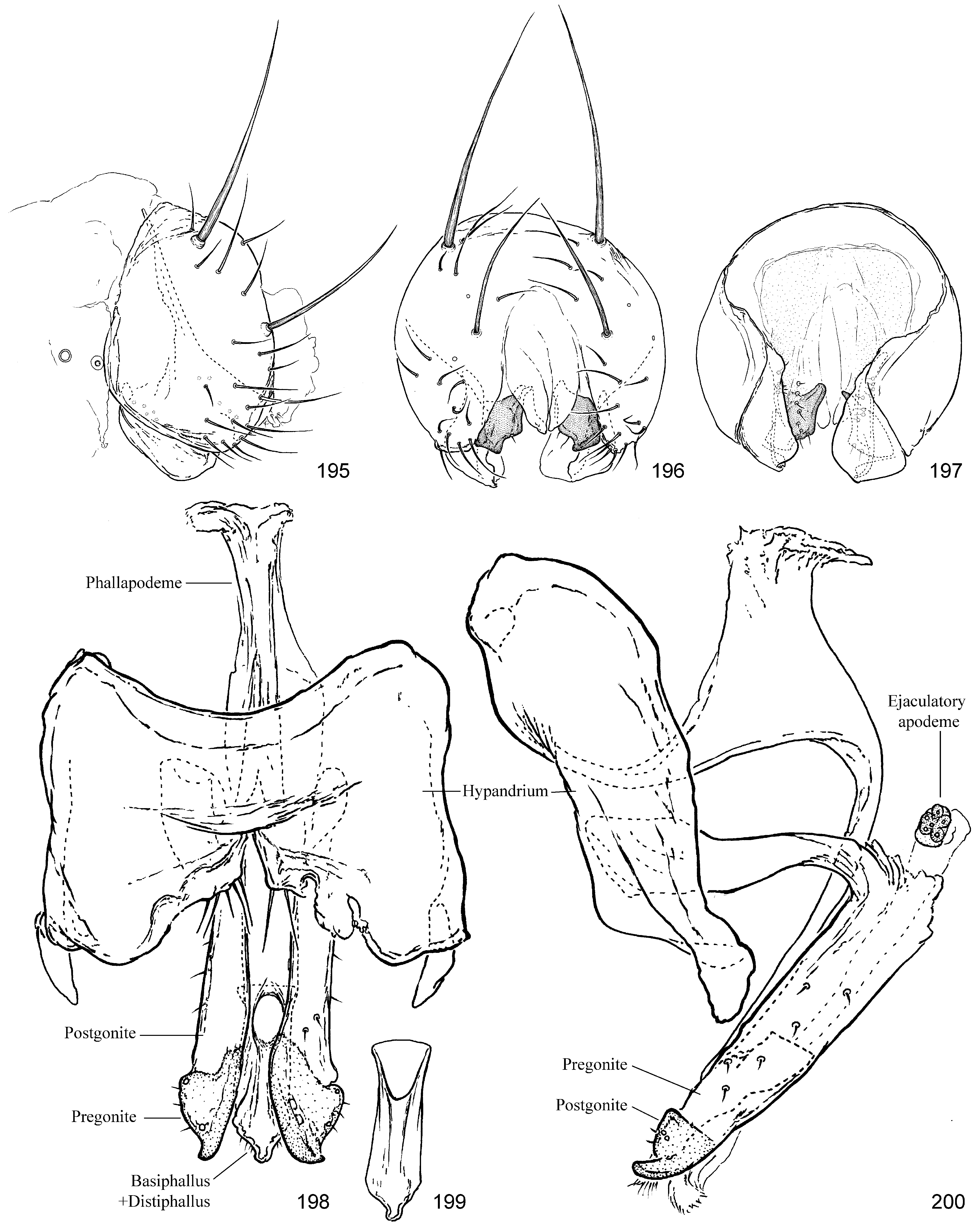
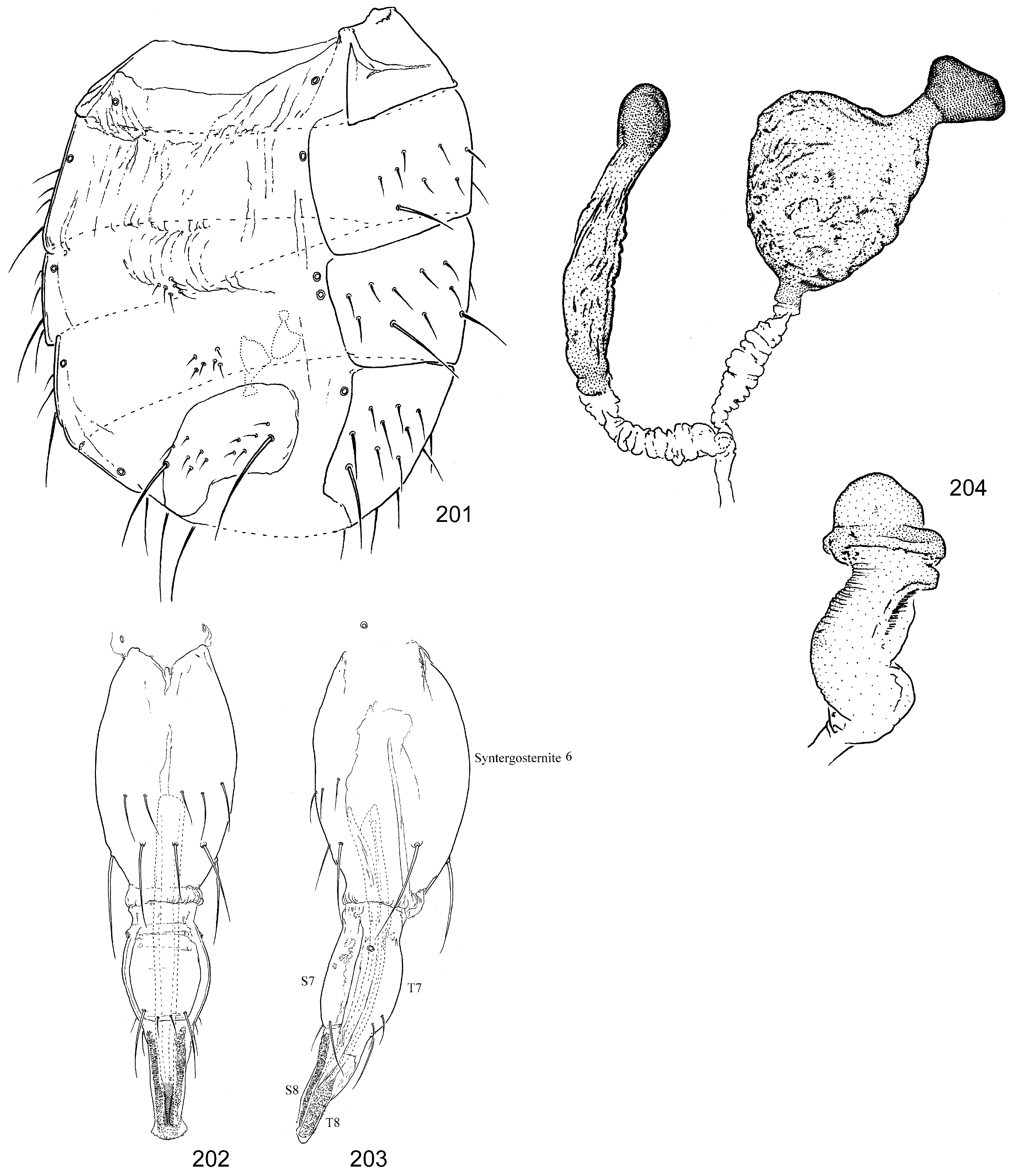
( Figs 191–204 View FIGURES 191–194 View FIGURES 195–200 View FIGURES 201–204 , 411 View FIGURES 411–422 )
Type genus: Fergusonina Malloch 1924: 337 View in CoL , by Tonnoir (1937: 129) [as subfamily “Fergusoninae”]. Type species of genus: Fergusonina microcera Malloch, 1924: 338 View in CoL , by original designation.
Fergusoninidae has 40 described species in the genus Fergusonina ( Purcell et al., 2016) , but Scheffer et al. (2017) identified 85 “putative species” in their study, and there are potentially upwards of hundreds of additional species awaiting discovery ( Scheffer et al., 2004; Purcell et al., 2013). Species can be difficult to differentiate, with diagnosis often depending on minutae of the male and female genitalia—see Taylor (2004). Molecular sequence data are currently being used to delimit and refine species boundaries and relationships for the Melaleuca -feeding Fergusonina ( Scheffer et al., 2004) . Purcell et al. (2016) developed a phylogeny correlating the structure of the sclerotized larval dorsal shield to gall type, and also supported the use of host and larval morphology in diagnosis. An updated molecular phylogeny of the family utilizing multiple and protein-coding genes is being developed ( Purcell et al., 2016; S. Scheffer, pers. comm.).
The family is Australasian in distribution, with species known from Australia, India, Papua New Guinea, the Philippines and New Zealand ( Harris, 1982; Taylor et al., 2007). The last keys to species were provided by Tonnoir (1937), who recognized 20 species at the time, and by Taylor (2004), who keyed species associated Melaleuca . A catalogue to Australian species was provided by Evenhuis (1989g). The complete mitochondrial genome of Fergusonina taylori Nelson & Yeates was published by Nelson, Cameron & Yeates (2011).
Biology. All species of Fergusonina for which biology is known are gall-feeders in the living tissue of Myrtaceae and are involved in an obligate mutualistic association with nematodes in the genus Fergusobia Currie ( Tylenchida : Neotylenchidae ). This represents the only recorded mutualism between flies and nematodes. This relation- ship was first discovered by Morgan (1933) and then described by Currie (1937) for taxa reared from Eucalyptus .
Eucalyptus species are host for most known Fergusonina , but flies have also been reared from over 65 species of Melaleuca , Leptospermum, Sygyzium and Metrosideros ( Purcell et al., 2013; Scheffer et al., 2017). Females select ovipositions sites in areas of new growth, specifically in the developing buds of shoots, inflorescences, flowers, leaves or stems of the host plant, and the number, location and morphology of the resultant galls appear to be characteristic for each Fergusonina / Fergusobia pair ( Currie, 1937; Giblin-Davis et al., 2003; Purcell et al., 2015). As a result, while multiple species pairs may be found on the same host plant, the galls of each are usually visually distinct ( Taylor, 2004). Galls may house a single larva (“unilocular”) or many larvae (“multilocular”), each in its own locule ( Purcell et al., 2015). Ye et al. (2007) reported galls containing tens to hundreds of larvae. Larvae in multilocular galls may have been laid by one or more females ( Purcell et al., 2015). As many as four species of fly can be found on a single host species, and one species of fly may be found on one or more species of host plant, usually within the same host subgenus, but sometimes in more distantly related hosts if those hosts are sympatric, possibly as a result of the availability of novel host choice via artificial plantings (Purcell et al., 2017).
Overall host associations largely appear to be conservative, with all species restricted to a single host genus in Scheffer et al. (2017), who analyzed fergusoninid phylogeny with respect to host usage and gall type. The authors found 73% of species on only a single host species, but they noted that this was a likely underestimate of monophagy in the group. Other studies found host usage among broad-leaved Melaleuca -feeders to be similarly conservative, where flies showed fidelity to only one or two host species ( Taylor, 2004; Scheffer et al., 2004). This is in contrast to the similarly plant-feeding Agromyzidae , whose relatively high levels of monophagy are likely only a result of limited sampling and likely to decrease substantially given further study.
There are 42 species of Fergusobia presently described ( Davies et al., 2010, 2016). The nematode-fly pairing is an exclusive relationship between one species of nematode and one species of fly ( Davies & Giblin-Davis, 2004), although the occurrence of multiple species pairings on the same host plant, and sometimes the same individual plant, allows for the theoretical possibility of the horizontal transfer of nematodes between fly species ( Purcell et al., 2015). Close patterns of co-evolution between the nematode, fly and host plant are evident, at least in some clades ( Davies & Giblin-Davis, 2004; Taylor et al., 2005; Nelson et al., 2011a, b), but much remains to be discovered of these complex relationships.
Adult female Fergusonina carry Fergusobia nematodes within their abdomens, the juveniles of which are deposited with fly eggs during oviposition on or near undifferentiated meristematic host plant tissue. The juvenile nematodes are the first to feed on the plant, inducing gall formation before the hatching of the fly egg ( Giblin-Davis et al., 2001). The gall is maintained by the fly and provides shelter and a food source for the fly and nematode alike, which feed on hypertrophied plant cells and secretions in the chamber it excavates ( Giblin-Davis et al., 2003).
As summarized in Purcell et al. (2015), the nematodes that are deposited on the host plant during oviposition develop into at least one generation of parthenogenetic females, which eventually lay eggs that become diploid females and males (the latter are also potentially haploid), producing the sexual, or amphimictic generation. The mated females of this generation are the preparasitic stage that invades the third instar of the fly larva, subsequently moulting without the development of a new cuticle. These female nematodes become fully parasitic, losing the stylet and digestive tract, and develop epidermal microvilli to absorb fly haemolymph. The eggs of this parasitic female hatch and move into the female host’s oviduct to be deposited with the next generation fly eggs in new plant tissue, completing the cycle ( Currie, 1937; Giblin-Davis et al., 2001). No male flies have ever been found with these nematodes present. Further details on life cycle, host specificity and diversity of Fergusobia nematodes are discussed in Davies et al. (2016), following a series of articles with nematode descriptions in Davies et al. (2014). A summary of Fergusonina / Fergusobia species associations, host associations and gall types, as influenced by fly oviposition placement and timing, were provided by Nelson et al. (2014).
Due to host specificity of the fly/nematode pair, Fergusonina turneri Taylor (paired with the nematode Fergusobia quinquenerviae Davies & Giblin-Davis ) were released as part of efforts to control Melaleuca quinquenervia in Florida, where the plant is considered an invasive weed and a severe threat to everglade ecosystems ( Pratt et al., 2013). Control efforts were considered unsuccessful, as viable populations did not establish following release events in 2005 and 2006.
Immature stages. The egg and all larval instars of Fergusonina nicholsoni Tonnoir were described by Currie (1937), who also discussed the third instars of 17 additional species. The third instar of F. syzygii Harris was described by Harris (1982). Hennig (1958) described the puparium and enclosed third instar of F. tillyardi Tonnoir. The egg of F. turneri was figured by Taylor (2004). Additional descriptions and/or photos and illustrations of the distinctive larvae of fergusoninid species are provided in Harris (1982), Taylor (2004), Taylor & Davies (2010), Nelson et al. (2011), Purcell et al. (2016, 2017) and the references therein. Most fergusoninid larvae have a species-specific “dorsal shield” of sclerotized spicules, bands or combs extending from the mesothorax to abdominal segment 7; both the larva and puparium have a comb-like plate between abdominal segments 1 and 2.
Adult Diagnosis. Very small, compact, mostly bright yellow flies with blackish patches, mostly black setae, and a strong, often black ovipositor ( Fig. 194 View FIGURES 191–194 ). Head characteristically broad and flattened anteriorly ( Fig. 193 View FIGURES 191–194 ), with small ventral face and antenna, and large parafacial and lunule. Setae sometimes not much larger than surrounding setulae; vibrissa present, 1–3 lateroclinate fronto-orbitals, 0–2 prescutellar acrostichals and 1–3 dorsocentrals near posterior margin of scutum. Veins R 4+5 and M 1 subparallel ( Fig. 411 View FIGURES 411–422 ). Costa with humeral break and sometimes with subcostal weakening; subcostal vein abbreviated, ending in R 1.
Adult Definition. Colour yellow with characteristic pattern of black pigmentation dorsally, including spot on ocellar tubercle, one to three pairs of stripes on scutum (sometimes very reduced), frequently black ovipositor, and extensive to reduced pigmentation on abdomen ( Figs 191, 192, 194 View FIGURES 191–194 ). Setae mostly black. Body length 1.6–2.8mm.
Chaetotaxy: 1 inner vertical; 1 outer vertical (sometimes long); 2–3 fronto-orbitals (lateroclinate); 1 ocellar; 1 postocellar (divergent to subparallel); vibrissa short. Frons with numerous scattered setulae that continue along parafacial in single row, usually also continuing onto gena and postgena, with some of these scattered and sometimes forming a relatively linear series under eye. 1 presutural intra-alar (uncommonly 2); 1 postpronotal; 2 notopleurals; 2 postsutural supra-alar; 1 postsutural intra-alar; 2–3 dorsocentrals (postioned posteriorly on scutum); 0–2 acrostichals (postioned posteriorly on scutum); 2–3 scutellars; 0 proepisternal; 1 anepisternal; 1 katepisternal (sometimes also with additional shorter seta). Setae sometimes not much larger than surrounding setulae. Body micropruinose. Fore femur with several outstanding dorsal and posteroventral setae, both angled posteriorly, and with long, thin ventrobasal seta (sometimes also on mid leg); hind femur usually with 1 outstanding anteroventral seta subapically, but sometimes with 2 or more; mid tibia with ventroapical seta.
Head. ( Fig. 193 View FIGURES 191–194 ) Antenna small, porrect, held against face within shallow cavity; pedicel with dorsal seam absent; first flagellomere small, rounded; arista pubescent, with dorsobasal insertion. Lunule very large; frons strongly curved downwards anteriorly to meet face; ocellar tubercle near vertex. Face well sclerotized, small; with medial carina that is sometimes partially expanded over antenna. Gena approximately half height of eye. Clypeus rounded; palpus subcylindrical; labium short and tapered apically with one small pair of setae.
Thorax. Precoxal and postmetacoxal bridges absent. Prosternum narrow, subrectangular with anterior width slightly greater. Greater ampulla absent. Scutellum flat to slightly convex dorsally, sometimes with surface wrinkled. Subscutellum present, small or slightly enlarged. Coxopleural streak absent.
Wing. ( Fig. 411 View FIGURES 411–422 ) Clear to greyish. Veins R 4+5 and M 1 subparallel. Costa extending to M 1, but often quite weak after R 2+3 and sometimes apparently terminating at R 4+5 ( Taylor, 2004). Medial and cross-veins sometimes weak, with dm-m sometimes incomplete or absent. M 4 ending at or near wing margin; CuA+CuP ending before wing margin. Vein bm-m incomplete to absent. Costa with humeral break, but sometimes also with subcostal weakening; subcostal vein abbreviated, ending in R 1. Cells cu p and bm very small. Calypter hairs moderately long.
Legs. Slender, short. Tarsomeres relatively short, with terminal 4 segments not longer than wide.
Abdomen. Sternites 1–4 small, sometimes desclerotized with chaetotaxy reduced, including bare S1; at least S5 with one pair of dominant lateral setae ( Fig. 201 View FIGURES 201–204 ). Spiracles 1–5 in membrane below tergites; 6 th spiracle in membrane anterior to segment in females, and 7 th spiracle enclosed anterolaterally in T7 on posteriorly angled tubercle; male 6 th spiracle anterior to epandrium in membrane laterally and left 7 th spiracle present near 6 th. T3 sometimes incomplete on left side, tapering to a point and with “orphaned” spiracle on that side adjacent to 4 th spiracle ( Fig. 201 View FIGURES 201–204 ).
Male genitalia. ( Figs 195–200 View FIGURES 195–200 ) Pregenital sclerites reduced to thin dorsal strip (possibly only remnant of S8) that may be completely membranous; essentially symmetrical. Epandrium with one or two pairs of dominant setae; ventral margin somewhat narrowed, approximating base of surstyli. Subepandrial sclerite mostly flat, weakly sclerotized and plate-like with deep ventromedial desclerotization and one pair of thick, sclerotized ventral lobes with irregular vertical row of short setae. Cerci small, lobate, largely fused. Surstylus small, lobate, curved in crosssection with anterior margins directed medially. Hypandrium broad, essentially planar with arms lateral; setae in concealed cluster at base of pregonite. Phallic plate absent. Phallapodeme flat, carinate, with one pair of anteromedial processes meeting inner-medial surface of hypandrium; broadly arched or U-shaped in profile. Pregonite long, band-like, fused to inner-medial surface of hypandrium, with scattered small setae along length. Postgonite short with pointed tooth-like apex; position apical to, and largely contiguous with postgonite; with short setae and empty sockets. Phallus (basiphallus+distiphallus) small, clear, tubular, with oblique ovate opening not reaching distal margin of postgonite; ventrobasal margin shortly before opening contiguous with phallapodeme base. Ejaculatory apodeme very small, globular, on very short, but relatively wide duct that barely exceeds arch of pregonite.
Female genitalia. ( Figs 201–204 View FIGURES 201–204 ) T6 and S6 completely fused into medially bulging sclerotized tube with few posteromedial and ventromedial setae sometimes arranged in transverse series, and larger row of larger setae completely encircling segment posteriorly; partially retracted into segment 5. T7 and S7 fused into oviscape with sutures sometimes still visible; segments distal to oviscape retracted. S8 and T8 divided medially, band-like and minutely textured. Segment 10 reduced to “internal process” of S10, which forms long, narrow, well-sclerotized stylet. Intersegmental membrane after segment 8 very elongate, basally with anteriorly directed spicules. Spermathecae (2) present (described for the first time here but noted as present in Taylor (2004)); flattened, pigmented, with shape subcircular to subtriangular, with short apical stem supporting small, dark apical bulb; ducts clear, short, fused before union with genital chamber. Ventral receptacle approximately as long as spermatheca, subcylindrical with shallow transverse wrinkles before wider subapical collar and rounded apical cap.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |