Sundathelphusa orsoni, Husana & Kase & Mendoza, 2015
|
publication ID |
https://doi.org/10.5281/zenodo.4504208 |
|
publication LSID |
lsid:zoobank.org:pub:549829D6-65A9-4D97-B15E-361755F7577B |
|
persistent identifier |
https://treatment.plazi.org/id/BD1787BC-FFA4-725B-FC7D-E9C82F2FF8E7 |
|
treatment provided by |
Carolina |
|
scientific name |
Sundathelphusa orsoni |
| status |
sp. nov. |
Sundathelphusa orsoni View in CoL , new species
( Figs. 1–4 View Fig View Fig View Fig View Fig )
Material examined. Holotype, ♂ (16.7× 14.2 mm) ( NMCR 39110 ), under rocks, near first cataract, 366 m a.s.l., Mambukal Mountain Resort, barangay Minoyan, Murcia town, Negros Occidental province, Negros I., Philippines, 10° 30.729'N, 123° 06.162'E, coll. JCE Mendoza, 26 December 2010 GoogleMaps . Paratypes: 1 ♂ (15.0× 12.5 mm) ( NMCR 39111 ), same data as holotype GoogleMaps ; 10 ♂ (8.8× 7.8 mm – 16.0× 13.5 mm), 5 ♀ (13.0×11.0 mm – 20.3× 16.9 mm) ( ZRC 2014.0238 View Materials ), under rocks, along banks and shallows, upstream of sixth cataract, 462 m a.s.l., Mambukal Mountain Resort, barangay Minoyan, Murcia town, Negros Occidental province, Negros I., Philippines, 10°30’20.9”N 123°06’28.1”E, coll. JCE Mendoza, 29 December 2011 GoogleMaps .
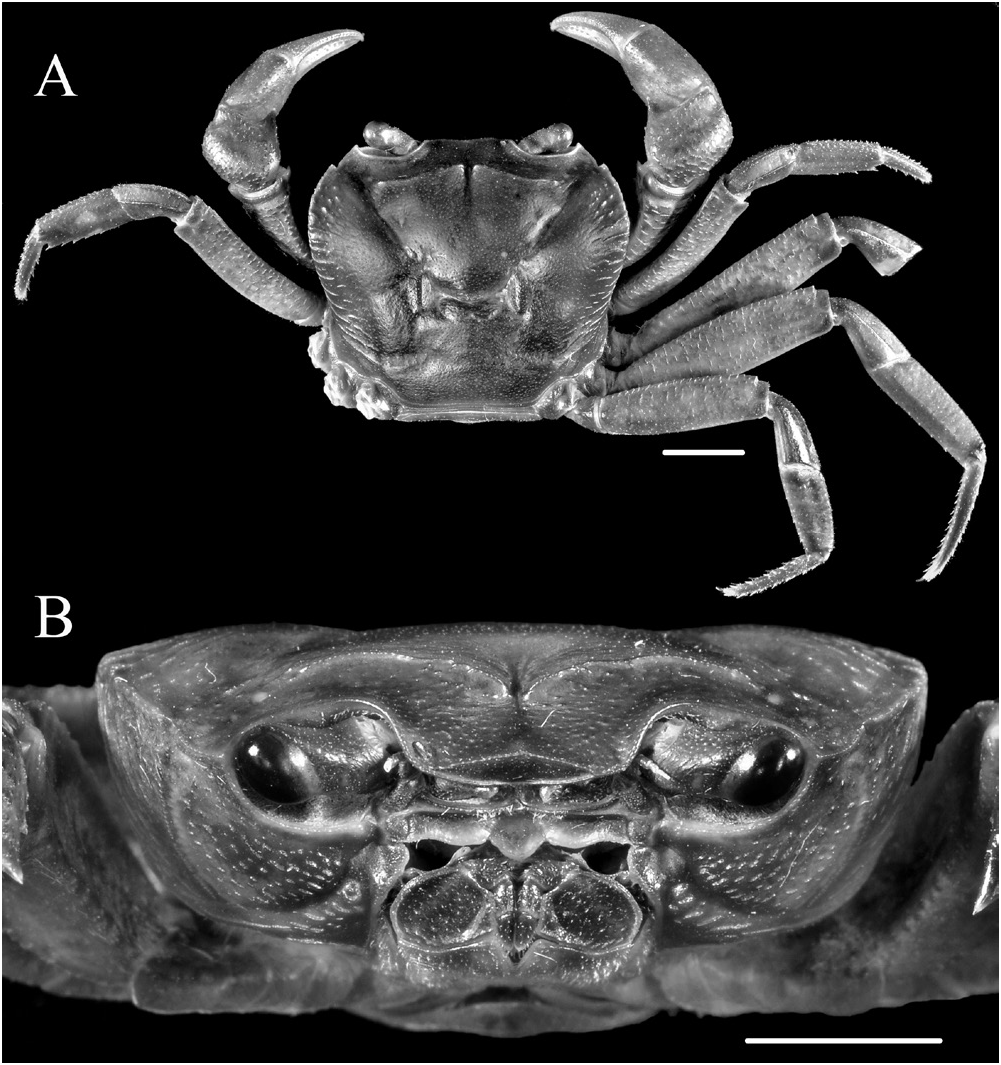
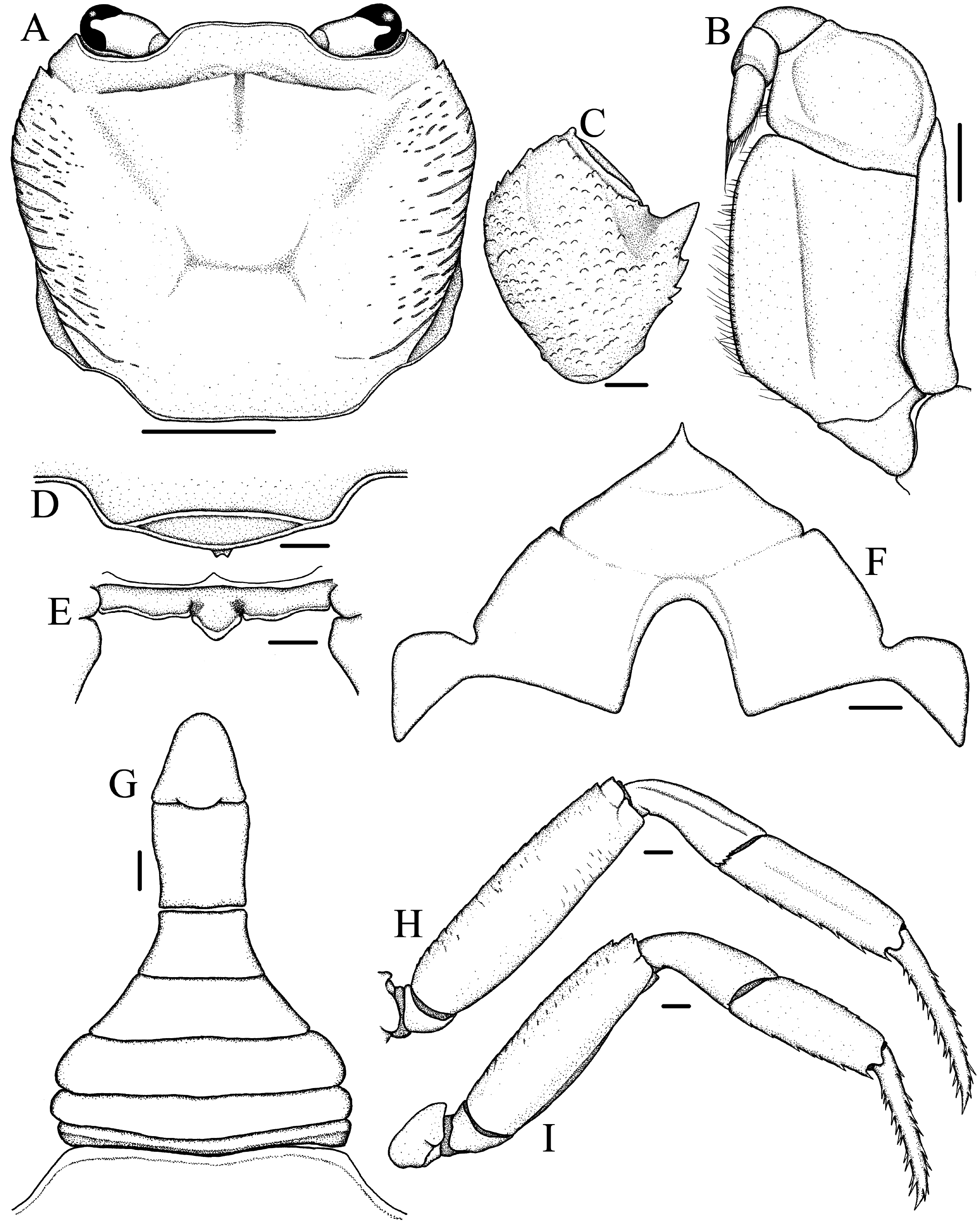
Description. Carapace ( Figs. 1A View Fig , 2A View Fig ) subquadrate in outline, about 1.1–1.2 times wider than long, widest at level of mesobranchial region; dorsal surface flattened longitudinally, transversely; branchial region moderately inflated, cardiac, intestinal regions flat; epigastric, postorbital cristae distinct, sharp, confluent; lateral regions with strong striae; distinct median groove between epigastric regions, cervical groove moderately deep, H-shaped gastric groove distinct. Front moderately broad, about 0.26–0.27 times greatest carapace width, gently deflexed ventrally; anterior margin with broad, shallow, median concavity in dorsal view; frontal median triangle ( Fig. 3D View Fig ) distinct, dorsal margin convex dorsally, lateral ends completely fusing with lateral margins. External orbital angle acutely triangular, external margin cristate, almost straight, distinctly longer than internal margin; epibranchial tooth small but distinct, triangular, well separated from external orbital tooth by V-shaped notch. Anterolateral margin cristate, gently convex, slightly serrated; posterolateral margin nearly straight, converging gradually towards posterior margin of carapace; central region of posterior margin slightly concave. Orbital margins cristate, supraorbital margin smooth, infraorbital margin serrated. Suborbital, sub-branchial, pterygostomial regions rugose, with several, prominent rows of small granules. Posterior margin of epistome ( Fig. 3E View Fig ) with protruding, triangular median lobe with rounded tip, separated from sinuous lateral lobes by distinct clefts.
Eyes ( Figs. 1C View Fig , 2B View Fig ) large, occupying almost entire orbit, corneas well developed. Third maxilliped ( Figs. 1C View Fig , 2B View Fig , 3B View Fig ) sparsely setose; ischium subrectangular, with distinct, oblique, submedian sulcus closer to mesial margin; merus subquadrate, anterior margin gently concave, lateral margin convex; exopod slender, tapering distally, external margin slightly sinuous, distal tip reaching level of mid-length of merus, flagellum well-developed, reaching slightly beyond mesial margin of merus.
Male thoracic sternum ( Fig. 1B View Fig , 3F View Fig ) broad, generally smooth except for slightly granular anterior region; sternites 1–4 fused, traces of sutures between sternites 2, 3 and 3, 4 as shallow depressions; sternite 4 with convex lateral margins, slightly raised area around anterior portion of sternoabdominal cavity. Sterno-abdominal cavity deep; press-button on sternite 5, midway between sutures.
Chelipeds, P1 ( Figs. 1A View Fig , 2A View Fig ), subequal, slender but relatively more robust in males; merus external (ventral) surface rugose with granular rows continuing onto posterior margin, anterior margin lined with large, conical granules; carpus ( Fig. 3C View Fig ) rugose with prominent rows of granules, inner angle with strong, sharply pointed tooth, followed by two smaller teeth posteriorly; palm slightly inflated, with minutely granular dorsal, external, internal surfaces; fingers granular, slender, cutting edges armed with several small teeth interspersed with slightly larger teeth, no molariform teeth, dactylus longer than superior margin of palm.
Ambulatory legs, P2–P5 ( Figs. 1A View Fig , 2A View Fig , 3H, I View Fig ), long, slender, P3 longest, total length (coxa-dactylus) about 1.8 times maximum carapace width, P5 shortest, total length (coxa-dactylus) about 1.6 times maximum carapace width; anterior margins of meri distinctly serrated, with low subdistal tooth, posterior margins smooth, cristate on all legs; carpi short, with longitudinal submedian ridge on dorsal surface except for P5, widened distally; propodi flattened, margins, particularly posterior, serrated with spiniform setae; dactyli slender, subequal in length to propodi, with several short, spiniform, marginal setae.
Male pleon ( Figs. 1B View Fig , 3G View Fig ) inverted T-shaped, relatively narrow for the genus; somite 1 thin, sinuous; somites 2–5 subtrapezoidal, progressively narrowing distally; somite 6 subrectangular, distal width slightly greater than proximal, median length greater than maximum width (distal end), lateral margins slightly concave subproximally; telson subtriangular, apex rounded, lateral margins concave, basal width equal to median length, distal tip reaching to level of posterior quarter of P1 coxae in ventral view.
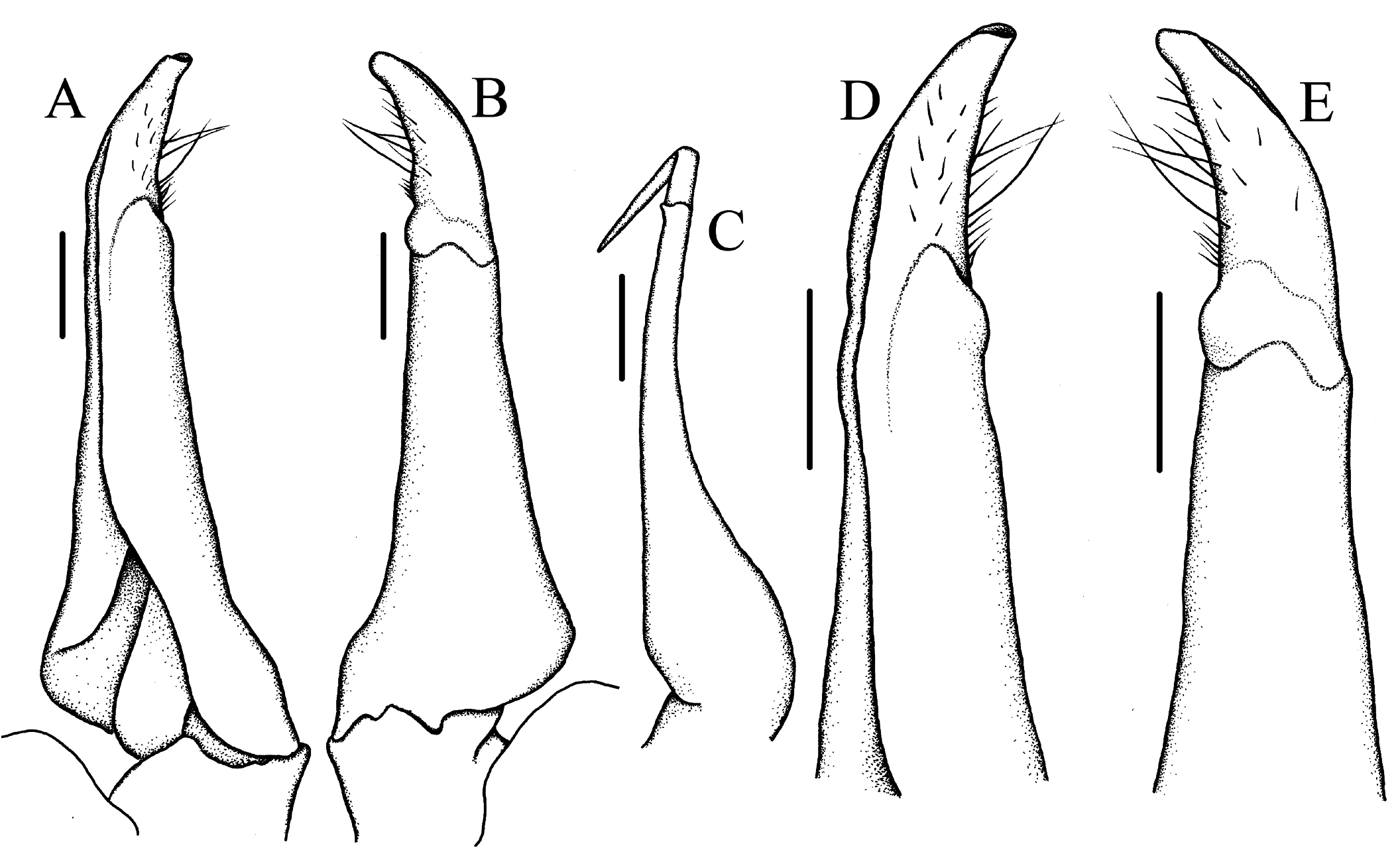
G1 ( Figs. 4A, B, D, E View Fig ) moderately slender, tapering slightly, subterminal segment generally straight, mesial margin nearly straight, lateral margin straight, steeply oblique; terminal segment distinctly curving laterally, about 0.43 times length of subterminal segment, subconical, distal tip moderately broad. G2 ( Fig. 4C View Fig ) slender, tapering distally; terminal segment long, about 0.38 times length of subterminal segment.
Colouration. The live colouration of Sundathelphusa orsoni , new species, is dark brown to brownish black on the dorsal carapace and ambulatory legs. The orbital margins and posterior margin of the epistome are yellow-orange, and stand out distinctly. The suborbital and pterygostomian regions, as well as the merus of the third maxilliped are similar in colour to the dorsal regions. The ischium of the third maxilliped, the sternum and pleon are pinkish gray. The chelipeds appear reddish brown throughout their entire length.
Etymology. The new species is named after Mr. Orson Ong, for the support and hospitality he extended to the third author on his visits to Negros, and whose help has been instrumental in the discovery of this new species.
Remarks. Sundathelphusa orsoni , new species, is counted among those Philippine species of Sundathelphusa which have a relatively narrow, subquadrate carapace and long, slender ambulatory legs, such as S. longipes ( Balss, 1937) , S. celer ( Ng, 1991) and S. holthuisi Ng, 2010 , from Luzon, and S. wolterecki ( Balss, 1937) from Mindanao.
Sundathelphusa orsoni is most similar to S. holthuisi ( type locality: Atimonan, Quezon, Luzon I.) in the general outline of the carapace and the G1. (cf. Ng, 1991: 15, figs. 1, 3A, B, 4; Ng, 2010: 569, figs. 2B, C, 3B, 4B, D, J–N). The two species can be distinguished, however, by the following morphological features: 1) the carapace is relatively narrower, with the lateral margins less convex, in S. orsoni ( Figs. 1A View Fig , 2A View Fig , 3A View Fig ) (relatively wider, lateral margins more convex, in S. holthuisi ; cf. Ng, 2010: fig. 2B, C); 2) the lateral margins and branchial regions of the carapace, as well as the suborbital and pterygostomian regions, are prominently striated with granular rows in S. orsoni ( Figs. 1A View Fig , 2A View Fig , 3A View Fig ) (much less striated in S. holthuisi ; cf. Ng, 2010: fig. 2C); 3) the notch separating the epibranchial tooth from the external orbital tooth is deeper and much more pronounced in S. orsoni ( Fig. 3A View Fig ) (shallower, hardly indenting the carapace anterolateral margin in S. holthuisi ; cf. Ng, 2010: fig. 2C); 4) the epigastric and postorbital cristae are sharper and better developed in S. orsoni ( Figs. 1A, B View Fig , 2A, B View Fig , 3A View Fig ) (weaker, lower in S. holthuisi ; cf. Ng, 2010: fig. 2C, 3B); 5) the ambulatory legs are relatively shorter and stouter in S. orsoni ( Figs. 1A View Fig , 2A View Fig , 3H, I View Fig ) (relatively longer and more slender in S. holthuisi ; cf. Ng, 2010: fig. 2B); 6) in the male pleon of S. orsoni , the lateral margins of the telson tend to converge distally, giving it a more tapered shape, and the sixth somite is longer, narrower with sinuous lateral margins ( Figs. 1C View Fig , 3G View Fig ) (telson relatively broader, lateral margins mostly parallel, sixth somite broader, lateral margins straight in S. holthuisi ; cf. Ng. 1991: fig. 4A; Ng, 2010: fig. 4D); and 7) the G1 is generally stouter, mostly straight, except for the strongly curved terminal segment which is also much shorter, in S. orsoni ( Fig. 4A, B, D, E View Fig ) (more slender, curving evenly from base to tip, terminal segment longer in S. holthuisi ; cf. Ng, 1991: fig.4B–E; Ng, 2010: fig. 4J–L, M). Furthermore, the two species differ in the live colouration. Whereas S. orsoni is mostly dark brown (JCEM, pers. obs.), S. holthuisi has been described to have a light-red or orange-red carapace and purple or red pereiopods ( Ng, 1991: 16).
Sundathelphusa orsoni , new species, can be readily separated from S. longipes ( Balss, 1937) (type locality: Philippines) and S. celer ( Ng, 1991) (type locality: Los Baños, Laguna, Luzon I.) by the morphology of the G1. The terminal segments of the G1 of the latter two species are not distinctly curved, as in S. orsoni . Also, there is a distinct hump or convexity on the lateral (external) margin, at the level of the joint between the terminal and subterminal segments, in both S. longipes and S. celer , which is absent in S. orsoni (cf. S. celer – Ng, 1991: figs. 5B–E; Ng, 2010: fig. 4E – G, H; and S. longipes – Ng, 2010: fig. 1H, I, J, K). As for the epibranchial tooth, in the male lectotype (cf. Ng, 2010: fig. 1A) and the female paralectotype (cf. Balss, 1937: fig. 15) of S. longipes and in the male holotype of S. celer ( Ng, 2010: fig. 2A) it is not as clearly separated from the external orbital tooth by a U-shaped notch, as it is in S. orsoni .
Sundathelphusa orsoni , new species, can also be distinguished from S. wolterecki ( Balss, 1937) ( type locality: Lake Lanao, western Mindanao) primarily by the condition of the external orbital and epibranchial teeth. In S. wolterecki , both are strongly projecting straight and towards the anterior and their tips are much more acute, whereas in S. orsoni , they are smaller, less projecting, and relatively more obtuse. Also, the ambulatory legs of S. wolterecki are relatively more slender than those of S. orsoni (cf. Balss, 1937: figs. 17, 18).
The type series were collected from a large stream winding through a forested area, which the local populace have turned into a resort and tourist area owing to the presence of hot springs (it is located at the northwestern foot of an active volcano, Mount Kanlaon). The area is also popular for its scenic waterfalls, of which there are several along the main stream. The holotype and one paratype were collected from under rocks in a small rivulet on a steep bank, which drains into the main stream located downstream from the first cataract. More specimens were collected further upstream in higher altitude beyond the sixth cataract under rocks in the shallow, riffle area of the stream, where some rocks were partly above the waterline. Little else is known about the biology of this species and more studies are warranted for its better understanding, especially considering the highly threatened status of much of the native fauna and flora of Negros Island due to extensive deforestation (see Heaney & Regalado, 1998).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |