Marcusenius devosi, Kramer & Skelton & Bank & Wink, 2007
|
publication ID |
https://doi.org/10.1080/00222930701250987 |
|
DOI |
https://doi.org/10.5281/zenodo.4658100 |
|
persistent identifier |
https://treatment.plazi.org/id/C512B407-FFD4-A04C-E7B8-1220FD5FD9B0 |
|
treatment provided by |
Carolina (2021-04-01 17:11:36, last updated 2021-04-01 17:59:55) |
|
scientific name |
Marcusenius devosi |
| status |
sp. n. |
Marcusenius devosi , sp. n.
( Figure 1 G View Figure 1 )
Type specimens. Holotype: SAIAB 79138 (specimen Ta13na), Kenya: Tana River . Paratypes: SAIAB 79139 (14), ZSM 35091 View Materials (3) , ZSM 35092 View Materials (1) , ZSM 35093 View Materials (4) , ZSM 35094 View Materials (7).
Type locality. Kenya: Lower Tana River near village Wenje : 1 u 52938.10S, 40 u 8922.50E (no. 14, Figure 4 View Figure 4 ) .
The presence of G. macrolepidotus , or a form of this species, in the equatorial Tana River of East Africa was suggested by Whitehead and Greenwood (1959) and Whitehead (1959, 1962); for M. macrolepidotus adopted by Seegers (1996, p. 76). However, a critical comparison with M. macrolepidotus has not been made.
Diagnosis. Longest mean pD of 41% (39.5–43.4%) of SL, shortest mean PAL of 59.1% (57–61%) of SL, shortest mean PDL of 62.3% (59.8–64%), long mean CPL of 20.2% (19.2–22.1%), median SLS of 62.5 (56–66) vs. 55.5 (52–62) in M. macrolepidotus , a median 22 (21–24) dorsal fin rays, 28 (26–30) anal fin rays, 16 (14–18) scales around caudal peduncle; EOD lacking weak head-negative pre-potential, strongest Namp relative to Pamp among samples from all origins (typically 137%), but short Ndur (about 140 Ms at 25 u C and ‘‘5% threshold criterion’’), Pdur of long duration (typically greater than 230 Ms), brief PNsep (typically 76 Ms). Fish from Rovuma River: anal and dorsal fin ray counts, one ray more each, SPc identical, CPD/CPL ratio significantly lower, LD and HL higher.
Description. Head with terminal mouth well in front of eye, mental lobe on lower jaw protruding beyond upper jaw. Head and body dorsolaterally compressed. Dorsal fin situated about two thirds of standard length from snout, obliquely orientated, anteriorly higher and posteriorly lower, distal margin sometimes only slightly crescentic with anterior two or three rays longer than posterior rays, number of rays 21 (N 53), 22 (N 518), 23 (N 55), 24 (N 54); anal fin opposite dorsal fin with distinctly more anterior origin, obliquely orientated, anteriorly lower and posteriorly higher, anterior rays longer than posterior ones, especially in males where they also appear stronger and often darkened, distal margin crescentic (in males only posterior to rounded, elongated anterior part of fin), number of rays 26 (N 51), 27 (N 59), 28 (N 514), 29 (N 54), 30 (N 52). Scales cycloid with reticulate striae, scales extending anteriorly to operculum and pectoral fins (beyond pelvics). Scales on caudal peduncle circumference, 14 (N 52), 15 (N 51), 16 (N 526), 17 (N 50), 18 (N 51). Caudal peduncle relatively deep, subcylindrical entire length, usually 20% (19–21%) in SL. Electric organ discharge biphasic lacking a weak pre-potential, Pamp of relatively long duration (around 230–235 Ms at 25 u C and ‘‘5% threshold criterion’’), increasing with SL at least in females and juveniles, Ndur of very short duration (usually around 140 Ms), but very strong Namp (137% of Pamp). No sexual dimorphism recognized. Males approaching sexual maturity develop a kink in the base of the anal fin which is absent in juveniles and females where the anal fin base is straight.
Colour in preservation. Head and back when seen from above, dark. Body sides light brown to light ochre, the ochre found especially on lower parts and underside. Dorsal fin usually darker than anal fin. Homogeneous coloration without any blotches, increasingly lighter from back to belly.
Ecology. The Tana River is a major, perennial river of about 700 km length that originates from the equatorial Mt. Kenya and flows into the Indian Ocean. In its final part close to the sea the river is bordered by gallery forest, surrounded by dry savannah on both sides. In August the water was murky and brown, and visibility very low. River borders are high and steep and difficult to climb up or down. Even though the water level was low the current was strong.
Distribution. Presently only known from the Tana River but range extension both to the north and south likely. Samples from the Rovuma River System more than 1000 km to the south are more similar to M. devosi than M. macrolepidotus in anatomical characters but take an intermediate, independent position in ISSR-PCR genetic analysis.
Relationships. Marcusenius devosi is considered closest to M. macrolepidotus based largely on the confusion of the identity of both species. Marcusenius devosi is distinguished most easily by its longer posterior body part relative to the anterior body part, as evidenced by higher lengths for the caudal peduncle and for pD, and lower lengths for PDL, PAL, and HL. BD was also lower in M. devosi . Marcusenius devosi when compared to M. macrolepidotus carries a longer mental lobe on the lower jaw, and has an EOD of shorter N phase duration and much greater strength relative to P phase (than Buzi specimens); the EOD of M. devosi shows no evidence for sexual dimorphism. There is also very little affinity with G. moeruensis whose morphological parameters are outside the range observed in the Tana River sample for: PDL, PAL, LD, pD, CPL, CPD, BD, nA, and SPc.
Etymology. Marcusenius devosi is named in honour of Dr. Luc De Vos, late curator of fishes at Nairobi Museum (born 8 December 1957 at Sint-Niklaas/ Belgium, deceased 14 June 2003 at Nairobi/ Kenya), for his contributions to African ichthyology and promotion of ichthyology in East Africa (see obituary by J. Snoeks).
Seegers L. 1996. The fishes of the Lake Rukwa drainage. Annales Sciences Zoologiques 278: 1 - 407.
Whitehead PJ, Greenwood PH. 1959. Mormyrid fishes of the genus Petrocephalus in Eastern Africa, with a redescription of Petrocephalus gliroides (Vinc.). Revue de Zoologie et de Botanique Africaine 60 (3 - 4): 283 - 295.
Figure 1. All species and forms of the Marcusenius macrolepidotus species complex, as studied in the present paper. (A) M. macrolepidotus (Peters, 1852), ZMB 3678 (lectotype L. Seegers; photo: L. Seegers). (B) Gnathonemus angolensis Boulenger 1905, BMNH 1905.5.29.64 (holotype). (C) Gnathonemus moeruensis Boulenger 1915, MRAC 14137 (holotype). (D) Gnathonemus pongolensis Fowler 1934, ANSP 54950 (holotype). (E) M. macrolepidotus (Peters 1852), SAIAB 060847, coll. R. Bills 1 Aug.1999, Lower Zambezi. (F) M. macrolepidotus (Peters 1852), SAIAB 060947, coll. R. Bills 15 Aug.1999, lower Pungwe River System. (G) M. devosi sp. n., coll. L. de Vos and B. Kramer 3/6 Sept. 2001, Lower Tana River/Kenya. (H) M. macrolepidotus (Peters, 1852), SAIAB 73790 (largest specimen), coll. R. Bills 14 Aug. 2003, Rovuma System. (I) M. macrolepidotus (Peters 1852), SAIAB 055874, coll. R. Bills 20 July 1997, Mulela River/Mozambique. (J) M. altisambesi sp. n., coll. F.H. van der Bank and B. Kramer, 11/12 August 2004, Okavango River, live fish of SL 13 cm photographed 20 April 2006. (K) M. altisambesi sp. nov, coll. F. H. van der Bank and B. Kramer, 21 August 1999, Upper Zambezi, Kalimbeza, live specimen of 16.5 cm SL photographed 3 July 2003. (L) M. macrolepidotus (Peters 1852), sampled together with SAIAB 67369, coll. R. Bills 29 Sept. 2002, Buzi River System, specimen of 13 cm SL photographed alive 22 February 2005. (M) M. pongolensis (Fowler, 1934), resurrected species, specimen of 11 cm SL reared in captivity from parents caught in Crocodile River, Incomati System, in February 1997, photographed alive 15 March 2005.
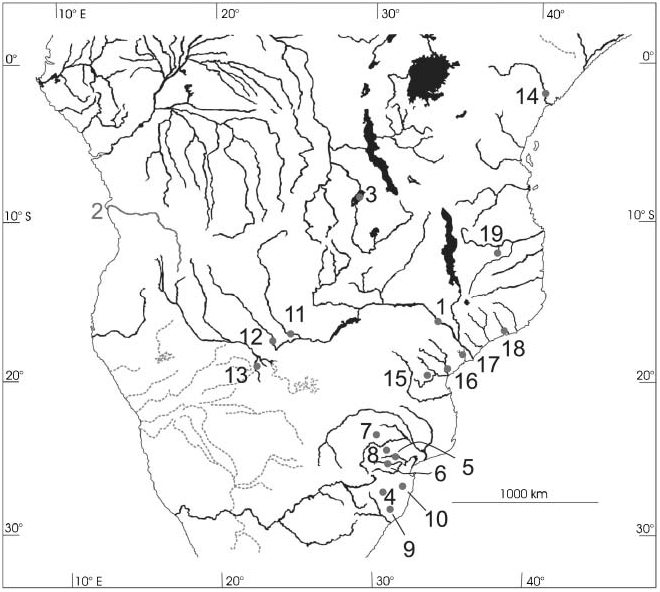
Figure 4. Partial map of southern Africa showing the sampling localities for Marcusenius sp. Only the larger rivers are shown. Numbers code for origins of samples: (1) Tete on the Lower Zambezi (M. macrolepidotus, as for types); (2) Quanza River (G. angolensis, type; exact sampling site unknown); (3) Lake Mweru (G. moeruensis, type); (4) Pongola River near Paulpietersburg (G. pongolensis, type); (5) Sabie River; (6) Crocodile River; (7) Groot Letaba River (Limpopo System); (8) Blyde River (Limpopo System; (9) KwaMaZulu stream, Mhlatuze System (not shown); (10) Pongola River, more downstream compared to (4); (11) Lisikili, Upper Zambezi; (12) Kwando River, (13) Okavango System; (14) Tana River; (15) Buzi River System; (16) Pungwe River; (17) Lower Zambezi, delta region; (18) Mulela River; (19) Rovuma River System.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |