Vernicia fordii, (Hemsl.) Airy Shaw (Hemsl.) Airy Shaw
|
publication ID |
https://doi.org/10.1016/j.phytochem.2019.112233 |
|
DOI |
https://doi.org/10.5281/zenodo.8302680 |
|
persistent identifier |
https://treatment.plazi.org/id/D66687DB-FFE5-0F7C-CA66-B727717BFCB8 |
|
treatment provided by |
Felipe |
|
scientific name |
Vernicia fordii |
| status |
|
2.2. Anti-neuroinflammatory effects of isolated components from V. fordii View in CoL View at ENA
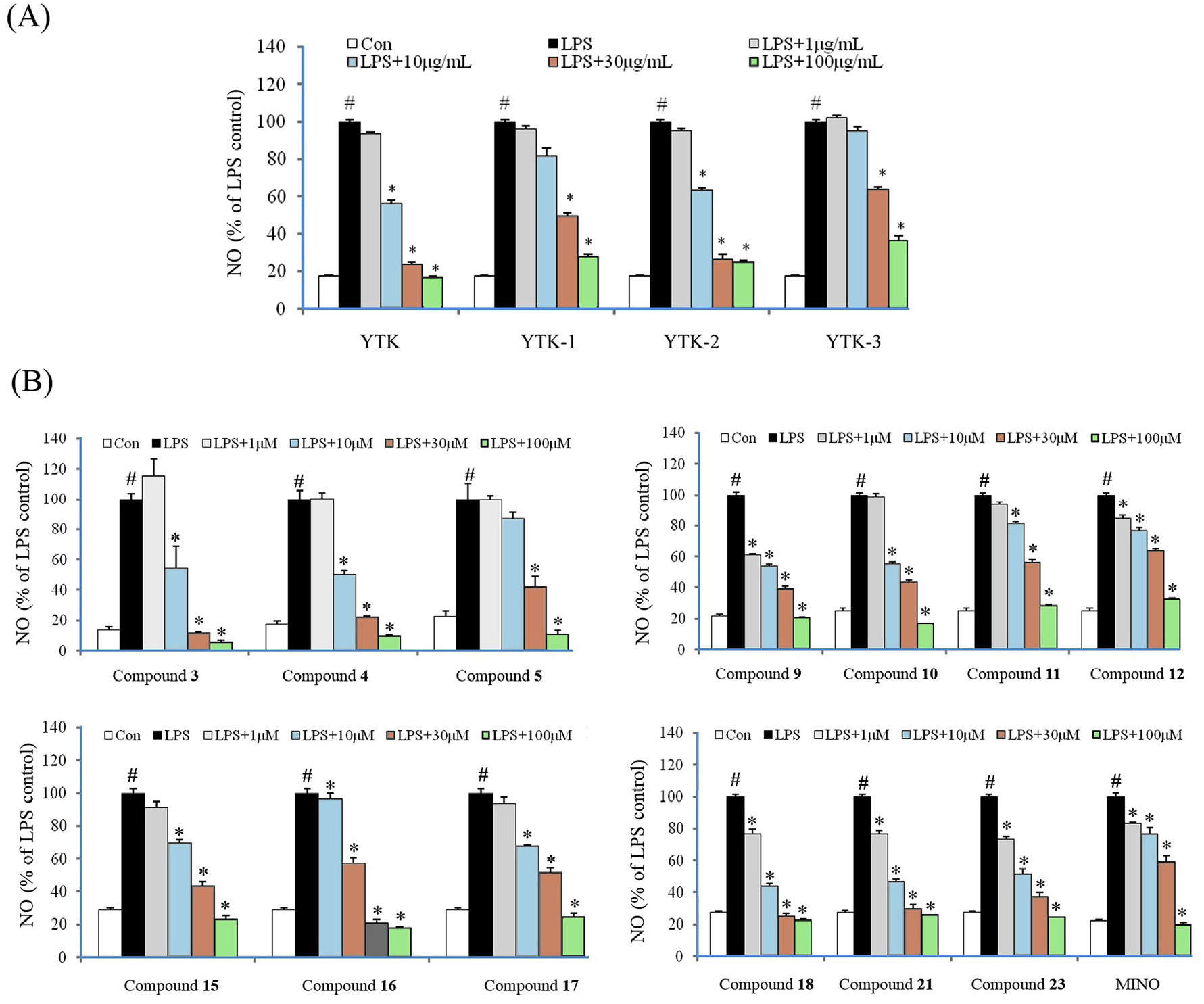
The extracts and identified components ( 1–23) were evaluated in vitro for their anti-neuroinflammatory activities in LPS-induced BV2 cells and the results are displayed in Table 5 View Table 5 and Fig. 8 View Fig . In order to avoid a false positive result, cell toxicities of the tested samples YTK: 70% ethanol crude extract; YTK-1: petroleum ether extract; YTK-2: ethyl acetate extract; YTK-3: n -butanol extract; Mino: minocycline; a the concentration for the extracts was μg/mL.
(supporting information Table S1 View Table 1 ) were assayed via MTT methods in normal BV2 cells. The 70% ethanol extract of the seed testa of V. fordii significantly inhibited the production of NO in LPS-activated BV2 cells with an IC 50 value of 7.03 μg/mL. Further assays indicated that the ethyl acetate extract (69.38%) with an IC 50 value of 10.70 μg/mL from the crude extract might be the bioactive fraction of the herbal.
Subsequently, components identified from the EtOAc extract were evaluated for their anti-neuroinflammatory activities. As a result, 13 lignans showed more potent inhibitory effects with IC 50 values from 1.28 to 18.47 μM than the positive control (minocycline, IC 50 = 37.04 μM). The effective ingredients were structurally characterized as dineolignans (compounds 3, 4, 5), sesquineolignans (compounds 9, 10, 16), monolignans (compounds 11, 12, 15, 17), and phenylpropanoids (compounds 18, 21, 23). Additionally, compounds 13, 14, 20, 22 elicited moderate anti-neuroinflammatory activities with IC 50 values ranging from 38.82 to 75.87 μM.
On the basis of the above-mentioned results, brief structure and activity relationships could be concluded as follows: firstly, the presence of 1, 4-bendioxanes enhanced the inhibitory effect against NO production, such as compounds 9 vs 11, 10 vs 12, and 15 vs 16.
Second, compound 13 substituted by a hydroxyl group at C-9′ exhibited moderate inhibitory activity while compound 14 with a methoxyl group at C-9′ displayed a significant inhibitory effect ( Table 5 View Table 5 ), indicating that the 9′-OMe enhanced the inhibitory activity of isoamericanol A.
In addition, for phenylpropanoids, such as compounds 18–22, both the reduction of C-3 hydroxyl or methoxylation of C-4 hydroxyl can increase inhibitory activities, such as compounds 18 vs 19 and 20 vs 22.
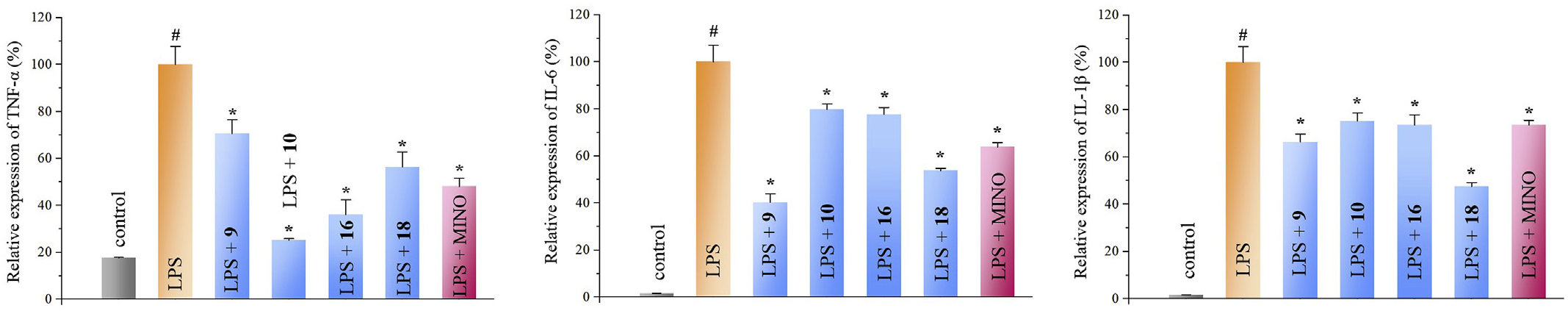
2.3. Effects of compounds 9, 10, 16 and 18 on LPS-induced overexpression of inflammatory mediators
Taking the inhibitory effects and amounts of the identified compounds 1–23, four principle bioactive components ( 9, 10, 16, and 18) were further investigated for the action mechanisms. Their effects on LPS-induced TNF-α, IL-1β and IL-6 overexpression in microglial cells were tested via the qRT-PCR method. The result revealed that compounds 9, 10, 16, and 18 significantly inhibited LPS-induced TNF-α, IL- 1β and IL-6 production at 10 μM as shown in Fig. 9. View Fig
3. Conclusion
Chronic neuroinflammation induced by over-activated microglia is considered to be a critical cause of the neurodegenerative disease. However, nonsteroidal anti-inflammatory drugs (NSAIDs) and estrogen, the most commonly used clinical drugs for neuroinflammation, can trigger severe side effects for patients with long-term medication. Therefore, effective natural neuroinflammatory inhibitors with low side effects can throw light on the development of therapy for neurodegenerative disease.
In this paper, a series of previous undescribed bioactive components ( 1–8), along with 15 known ones, from V. fordii were reported in this paper. The lignans from V. fordii were mainly composed of two types of substructures, benzodioxane and bistetrahydrofuran. The dineolignans ( 3–5) were composed of two 1, 4-benzodioxane units and one bistetrahydrofuran moiety; the sesquineolignans 9 and 10 were formed via one 1, 4-benzodioxane fragment and one bistetrahydrofuran unit; and compound 16 was comprised of two 1, 4-benzodioxane units.
The in vitro assay showed that most of these isolated components ( 3–5, 9–18, and 20–23) from V. fordii had anti-neuroinflammatory activities, suggesting that V. fordii has a great potential in the treatment of neuroinflammation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |