Thoropa bryomantis, Assis & Lacerda & Guimarães & Peixoto & Luna & Feio, 2021
|
publication ID |
https://doi.org/10.11646/zootaxa.4995.3.6 |
|
publication LSID |
lsid:zoobank.org:pub:97C7612C-0D55-426E-898A-BFBD71C83ABB |
|
persistent identifier |
https://treatment.plazi.org/id/DC40BC51-4D40-FFF1-ABFF-A22B604EFC7F |
|
treatment provided by |
Plazi |
|
scientific name |
Thoropa bryomantis |
| status |
sp. nov. |
Thoropa bryomantis sp. n.
( Figs. 1–6 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 )
Thoropa lutzi View in CoL — Bokermann (1965: 526, 530 [Figure 14], 535), in part; Cocroft & Heyer (1988: 216 [ Figure 5 View FIGURE 5 ]), in part; Izecksohn & Carvalho-e-Silva (2001: 88), in part; Carvalho-e-Silva & Carnaval (2004: 1, 2 [distribution map], 3), in part; Gasparini et al. (2007: 76, 77, 80, 86 [photography]); Almeida et al. (2011: 544, 548, 552 [ Figure 5F View FIGURE 5 ]); Haddad et al. (2013: 175 [photography, distribution map]), in part; Nunes-de-Almeida et al. (2016: 1, 2, 3 [distribution map], 4, 5, 6 [ Figure 2A View FIGURE 2 ], 7 [ Figure 3 View FIGURE 3 ], 8, 9), in part; Sabbag et al. (2018: 143 [ Figure 1 View FIGURE 1 ], 144, 146, 149), in part.
Thoropa cf. lutzi View in CoL — Sabbag et al. (2018: 147 [ Figure 3 View FIGURE 3 ], 149 [ Figure 4 View FIGURE 4 ], 150, 154).
Thoropa aff. lutzi View in CoL — Ferreira et al. (2019: 143, 152).
Holotype. MZUFV 13378 ( Figs. 1 View FIGURE 1 , 6A View FIGURE 6 ), adult male, collected on 22 December 2012 by C.L. Assis at Serra do Sapecado ( 21º14’13.08” S, 42º44’08.04” W; 758 m above sea level [a.s.l.], datum WGS84), Glória District , Cataguases Municipality, Minas Gerais State ( MG), Southeast Brazil. GoogleMaps
Paratypes. MZUFV 13377 , adult male, and UFMG 16459 View Materials , adult female, collected with the holotype . MZUFV 13820– 821 adult males, collected on 03 February 2013 by C.L. Assis and R.N. Feio . MZUFV 15726 , adult female, and MZUFV 14466 , adult male, collected on 05–06 December 2013 by C.L. Assis . MZUFV 16626 adult male, collected on 04 December 2015 by C.L. Assis; E . T. Silva and J . V.A. Lacerda .
Referred specimens. Pedra Elefantina, Antônio Prado de Minas Municipality ( 20°58’33.03” S, 42° 9’56.88” W; 737 m a.s.l., datum WGS84), Minas Gerais State, Southeast Brazil: UFMG 16460–461 View Materials and MZUFV 13824– 829 , 13837–839 , adult males, collected on 29 January 2013 by J . V.A. Lacerda, M.A. Peixoto, and C.S. Guimarães ; MZUFV 16670 , 16672 , adult males, collected on 09 January 2016 by C.L. Assis ; MZUFV 18374–375 , adult males, collected on 05 February 2017 by C.L. Assis. Parque Nacional do Caparaó, Alto Caparaó Municipality , Minas Gerais State, Southeastern Brazil : MZUSP 57952–953 View Materials , adult males, collected on 29–30 November 1980 by W . R. Heyer. Santa Teresa Municipality, Espírito Santo State, Southeastern Brazil : MNRJ 1373 View Materials , adult male, collected on 24–31 November 1942 by A. Ruschi. Muniz Freire Municipality, Espírito Santo State, Southeast Brazil : MNRJ 26159 View Materials , adult female, collected on 16 September 2000 by J.L. Gasparini .
Diagnosis. We included the new species in the genus Thoropa based on the following combination of anatomic characters: tympanum externally visible; absence of meniscus in eyes of adults (tympanum not externally visible and meniscus in eyes of adults present in Cycloramphus and Zachaenus ; Lynch 1971, Colaço et al. 2020), and absence of inguinal macroglands (inguinal macroglands present in Cycloramphus ; Lynch 1971, Heyer 1983). Among species of Thoropa , the new species can be diagnosed by the following combination of characters: (1) males SVL 19.6–23.5 mm, n = 24; (2) females SVL 23.9–25.9 mm, n = 3; (3) body slender; (4) dorsal color pattern with different tones of brown on a green background; (5) relative lengths of fingers I <II <IV <III; (6) dark colored nuptial pads with two elliptical segments on Finger I (one next to internal side of finger, and other on external surface of internal metacarpal tubercle); (7) nuptial pads with cone-shaped papillary epidermal projections inclined toward finger base; (8) papillary epidermal projections with a diameter of 53.1–91.6 μm, n = 106 PEPs of four males; (9) density of papillary epidermal projections 14–32 PEPs/mm 2, estimated in four males; (10) external carpal tubercle larger or equal in size to internal carpal tubercle; (11) head longer than wide; (12) snout rounded in dorsal view; (13) forearm of males not hypertrophied; (14) vocal slits present; (15) vocal sac single, subgular (16) advertisement call with harmonic structure; (17) call duration 0.23– 0.42 s; and (18) call peak frequency 2060–4470 Hz.
Comparison with large-sized species of Thoropa . Thoropa bryomantis sp. n. can be promptly distinguished from the large-sized species T. miliaris , T. taophora , T. saxatilis , T. megatympanum (characteristics in parentheses) by: male SVL 19.6–23.5 mm [n = 24] (range of SVL 31.1–102.1 mm for males of the other species; Caramaschi & Sazima 1984, Cocroft & Heyer 1988, Feio et al. 2006); females SVL 23.9–25.9 mm [n = 3] (range of SVL 37.8–85.9 mm for females of the other species; Caramaschi & Sazima 1984, Cocroft & Heyer 1988, Feio et al. 2006); body slender (body robust; Caramaschi & Sazima 1984, Cocroft & Heyer 1988, Feio et al. 2006); relative lengths of fingers I <II <IV <III (II <IV <I <III; Caramaschi & Sazima 1984, Cocroft & Heyer 1988, Feio et al. 2006); nuptial pads on Finger I (nuptial pads reaching fingers II and III; Caramaschi & Sazima 1984, Cocroft & Heyer 1988, Feio et al. 2006, Rebouças et al. 2017); dorsal color pattern with different tones of brown on a green background (dorsal color patterns with black to dark brown blotches over a gray to light brown background in T. miliaris , two dark to reddish grey blotches over a gray to light brown background in T. taophora , predominantly with dark blotches over a greenish-yellow background in T. saxatilis , and predominantly with dark brown blotches and bars on a yellowgreen background in T. megatympanum ; Caramaschi & Sazima 1984, Cocroft & Heyer 1988, Feio et al. 2006); nonpulsed advertisement call with harmonic structure (pulsed advertisement call without harmonic structure; Nunes-de- Almeida et al. 2016); presence of vocal slits and vocal sac (absence of vocal slits and vocal sac).
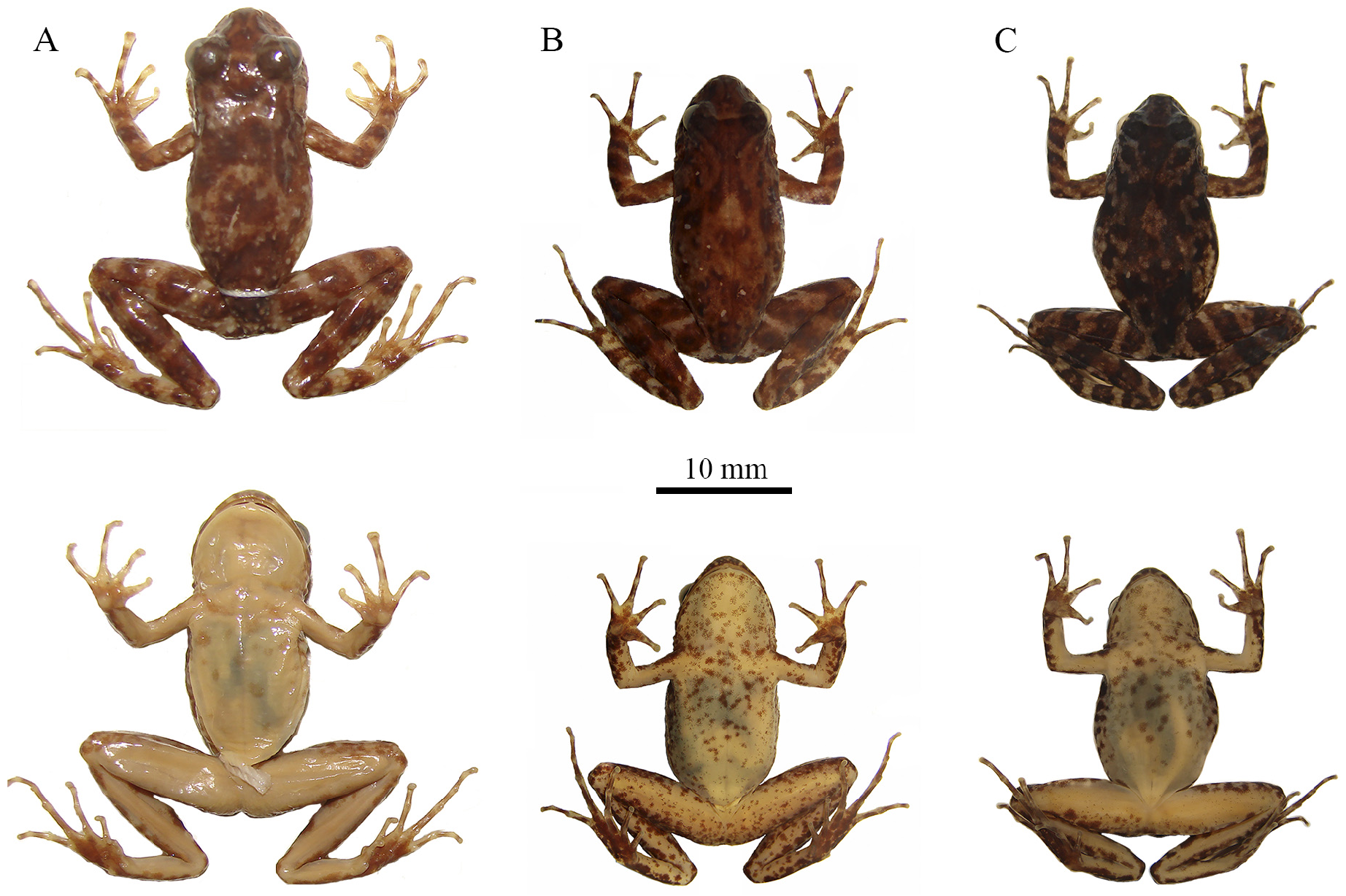
Comparison with small-sized species. Thoropa bryomantis sp. n. differs from T. lutzi (characteristics in parentheses) by: body slender (body robust) ( Figs. 2 View FIGURE 2 , 3 View FIGURE 3 ); snout rounded in dorsal view (snout truncated in dorsal view); palmar tubercle larger or equal in size to the thenar tubercle (palmar tubercle smaller than thenar tubercle); forearm of males not hypertrophied (forearm of male hypertrophied) ( Fig. 3 View FIGURE 3 ); nuptial pads with larger papillary epidermal projections, diameter ranging 53.1–91.6 ± 7.8 μm for 106 PEPs of four males, at a lower density ranging 14–32 ± 5.9 PEPs/mm 2 (diameter of papillary epidermal projections ranging 24–45 ± 4.3 μm in 96 PEPs of one male, density ranging 46–83 ± 14.8 PEPs /mm 2 estimated in two males) ( Figs. 4A–F View FIGURE 4 ). Advertisement call with longer duration, ranging 0.23– 0.42 s; higher peak of dominant frequency, ranging 2060–4470 Hz; higher minimum frequency, ranging 1890–2411 Hz; and higher maximum frequency, ranging 4134–6540 Hz; Nunes-de-Almeida et al. (2016) and the present study (shorter duration, ranging 0.18– 0.20 s; lower peak of dominant frequency, 1723 Hz; lower minimum frequency, ranging 517–1033 Hz; and lower maximum frequency, ranging 2928–3100 Hz; Nunes-de-Almeida et al. 2016).
The new species differs from Thoropa petropolitana (characteristics in parentheses) by the presence of nuptial pads on the inner side of the Finger I and on the internal carpal tubercle (nuptial pads only on the inner side of the Finger I and absent on the internal carpal tubercle; Bokermann 1965); nuptial pads with cone-shaped papillae (nuptial pads with spine-shaped papillae; Luna et al. 2018). The advertisement call of the new species differs in its harmonic structure; longer duration, ranging 0.23– 0.42 s; and lower minimum frequency, ranging 1890–2411 Hz; Nunes-de-Almeida et al. (2016) and the present study (pulsed structure; shorter duration, ranging 0.034 – 0.037 s; and higher minimum frequency, ranging 2250–3000.0 Hz; Nunes-de-Almeida et al. 2016).
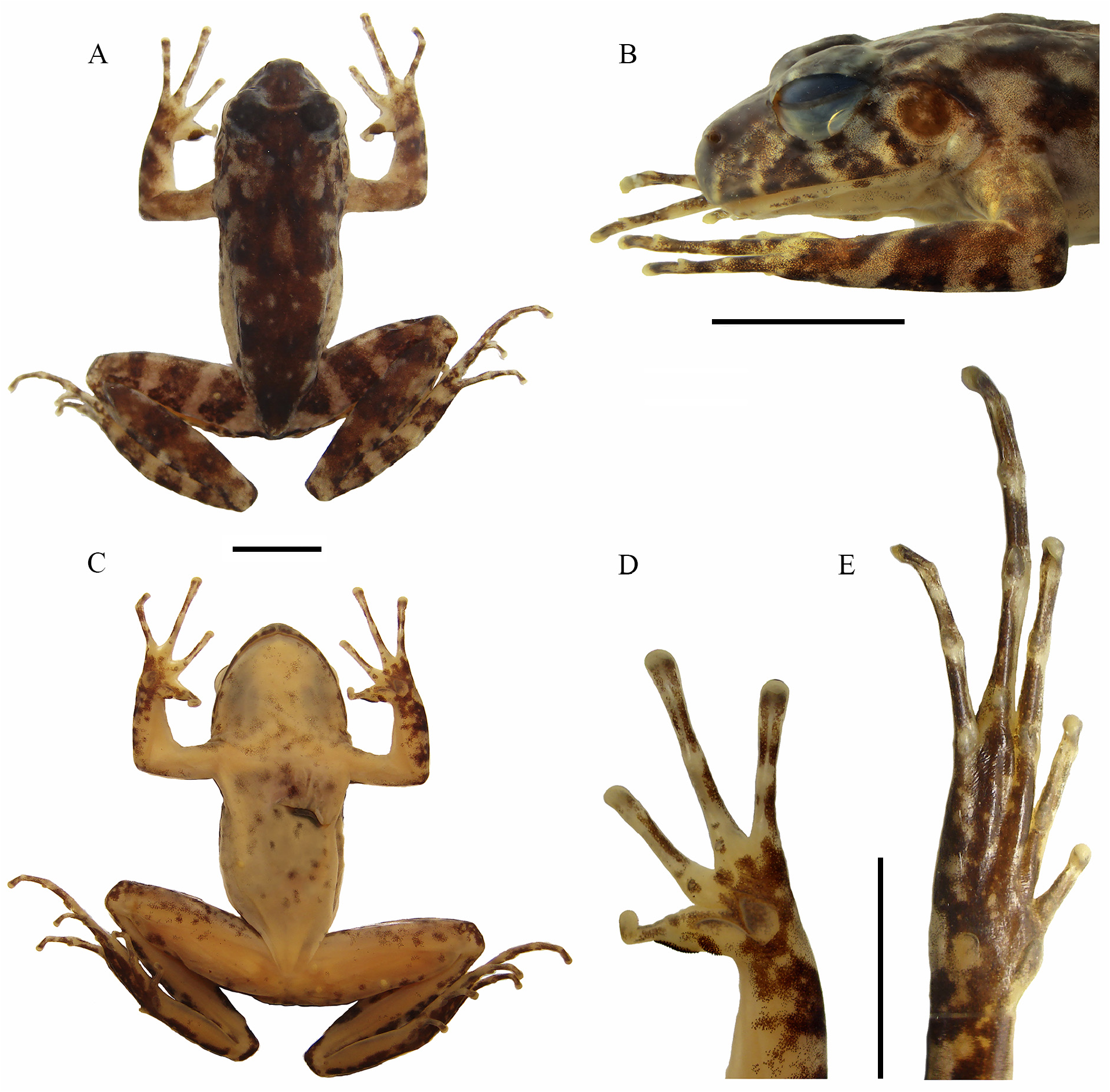
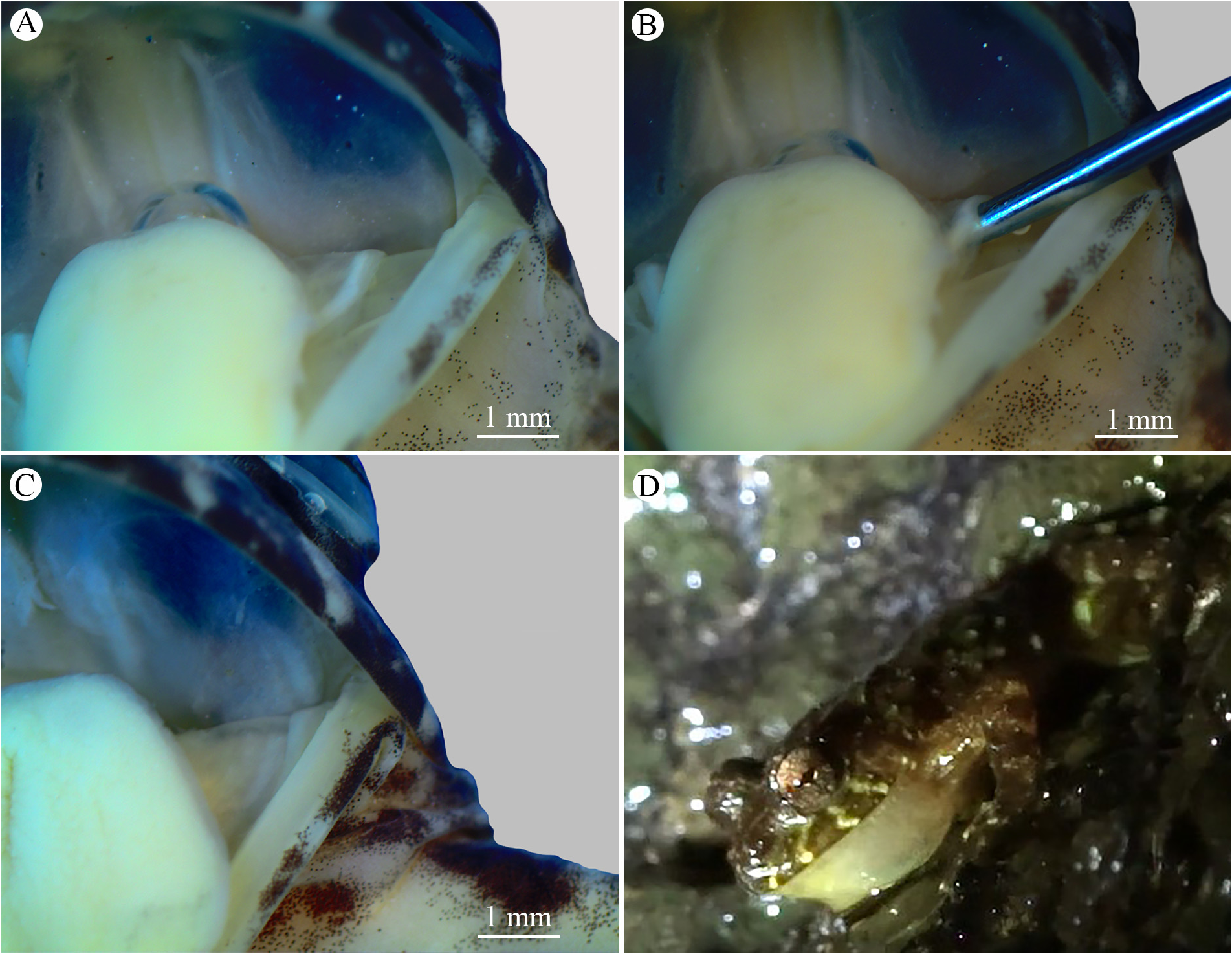
Description of holotype. Body slender and small (SVL 22.4 mm). Head longer than wide (HW 91.5% of HL). Snout shape rounded in dorsal and lateral views, ( Fig. 1A–C View FIGURE 1 ). Eyes prominent, laterally positioned, and directed slightly forward. Pupil elliptical, horizontal. Absence of meniscus at top edge of iris. Interorbital distance smaller than eye diameter (IOD 74.3% of ED). Nostril dorsolateral, slightly prominent, closer to snout than eye. Internarial distance smaller than eye-nostril distance (IND 90.3% of EN). Canthus rostralis slightly concave. Tympanum distinct, annulus timpanicus evident with diameter smaller than eye diameter (TD 74.7% of ED). Supratympanic fold pronounced, starting behind eye, marginating the annulus timpanicus, ending near arm insertion. Pseudodontoid on mandibular symphysis, protuberant. Pseudodontoid accommodated by round space between premaxillae when mouth is closed. Vocal slits present, longitudinal, originating on sides of the tongue. Vocal sac single, subgular, slightly distensible, evident only during vocalization. Vomerine teeth V-shaped in two oblique series between choanae, clearly separated medially. Choanae elliptical, lateral. Forearm not hypertrophied, shorter than upper arm length (FAL 94.4% do UAL). Fingers long with poorly dilated extremities ( Fig. 1D View FIGURE 1 ). Relative lengths of fingers: I <II <IV <III. Subarticular and palmar tubercles slightly prominent. External carpal tubercle rounded, internal carpal tubercle elongated, both similar in size. Dark colored nuptial pads positioned in two elliptical segments: one next to internal side of Finger I; the other, smaller, on external surface of internal metacarpal tubercle ( Fig. 1D View FIGURE 1 ). Nuptial pad surface easily distinguishable by the presence of numerous dark-colored cone-shaped papillary epidermal projections; axis leaning towards finger base. Hind limbs long; thigh slightly shorter than tibia (THL 95.6% of TL). Sum of tibia and thigh lengths longer than SVL. Feet with elongated toes, without dilation at tips, fringes absent ( Fig. 1E View FIGURE 1 ). Relative lengths of toes: I <II <V <III <IV. Inner metatarsal tubercle oval, more developed than external metatarsal tubercle; subarticular tubercle present, rounded. Cloacal opening at upper level of thighs. Granulations concentrated in dorsal, dorsolateral, tibial, and tarsal regions, absent in cloacal region. Skin of venter, gular region, and internal part of limbs smooth.
Coloration in life. Four dark brown spots on either side of upper lip, delimited by a greenish background; first three spots forming a triangular shape, with top toward the eye. Supratympanic fold dark brown. Tympanum light brown with central part greenish. Dark stripe from snout to eyes, covering nostrils and canthus rostralis. Iris light cream in color with horizontal reddish stripe and dark brown reticulations ( Fig. 5 View FIGURE 5 ); two dark spots at inferior and superior parts of pupil. Venter light cream in color with abdominal region whitish and remaining areas light pink. White and dark gray blotches distributed throughout pectoral, abdominal and gular regions and ventral edges of inner thigh. Palmar and plantar regions of limbs pigmented dark brown. Dorsolateral region with small white spots and dark brown spots on greenish background ( Fig. 5 View FIGURE 5 ). Cloacal region with horizontal black blotch. Dorsal background coloration light green with dispersed dark to light brown blotches. Dark brown inverted triangular blotch between eyes, bordered by light cream-colored line. Base of triangle connects eyelids while apex merges with a bifurcated dark blotch (inverted V-shaped figure) in middle of dorsum. Elongated light cream-colored spot in middle of dorsum. Posterior half of dorsum covered by large dark-brown blotch. Dark-brown transversal bar and spot, in post-sacral region. Three well-defined transversal dark brown stripes on dorsum of thigh, tibia and forearm; four dark brown stripes on tarsus-foot.
Coloration in preservative. Blotches of dorsum dark gray over light gray background. Stripes of thigh, tibia, forearm, and tarsus-foot and triangular spots on upper lip same color as dorsum. Venter whitish to pale cream in color, with dark and light blotches.
Measurements of holotype (mm). SLV 22.4, HL 8.2, HW 7.5, ED 2.2, IOD 1.6, IND 1.8, EN 2.0, TD 1.6, HAL 7.3, FAL 4.5, UAL 4.8, THL 12.3, TL 12.9, and FL 18.0.
Intraspecific variation. Variation in measurements is shown in Table 5. Males are smaller than females. Nuptial pads and vocal slits are absent in females ( Fig. 6A–C View FIGURE 6 ). Skin granulations vary in size and density. Most specimens (n = 19) possess two prominent tubercles next to the oral commissure. The external carpal tubercle might be larger or equal in size to the internal carpal tubercle, and rounded or elongated. Toe V might be smaller or equal in size as Toe III. Dorsal coloration varies in intensity; blotch in sacral region can be continuous with the blotch of the middle of the dorsum or distributed as smaller blotches. Vocal sac pigmentation varies in intensity ( Fig. 6D View FIGURE 6 ). Blotches on venter vary in size, density, and color intensity.
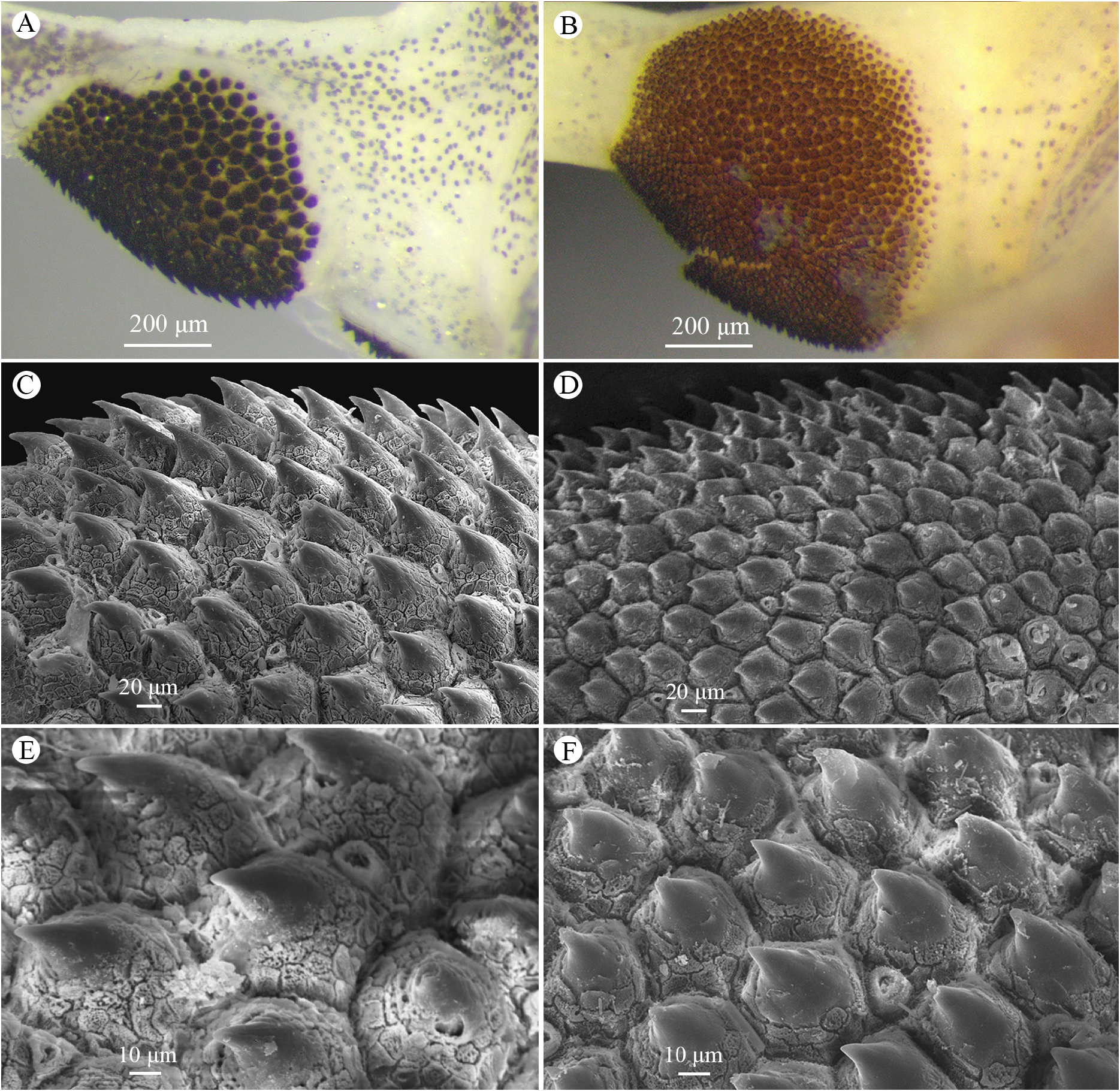
Nuptial pad. The surface of the nuptial pad is keratinized and readily distinguished by the presence of numerous papillary epidermal projections (PEPs) ( Fig. 4A View FIGURE 4 ). It is divided in two elliptical segments, one on the internal side of Finger I, and the other, smaller, on the external surface of the internal metacarpal tubercle. The PEPs are dark in color, and cone shaped with the axis leaning toward the finger base. ( Figs. 4A, C View FIGURE 4 ). The PEPs are not ornamented ( Fig. 4E View FIGURE 4 ), but possess pores between their bases, most having an elliptical shape and a well-defined border ( Figs. 4C, E View FIGURE 4 ). The diameter of the PEPs ranges 53.1–91.6 μm (n = 106 PEPs of four males), and their density ranges 14–32 PEPs/mm 2 (estimated density for four males).
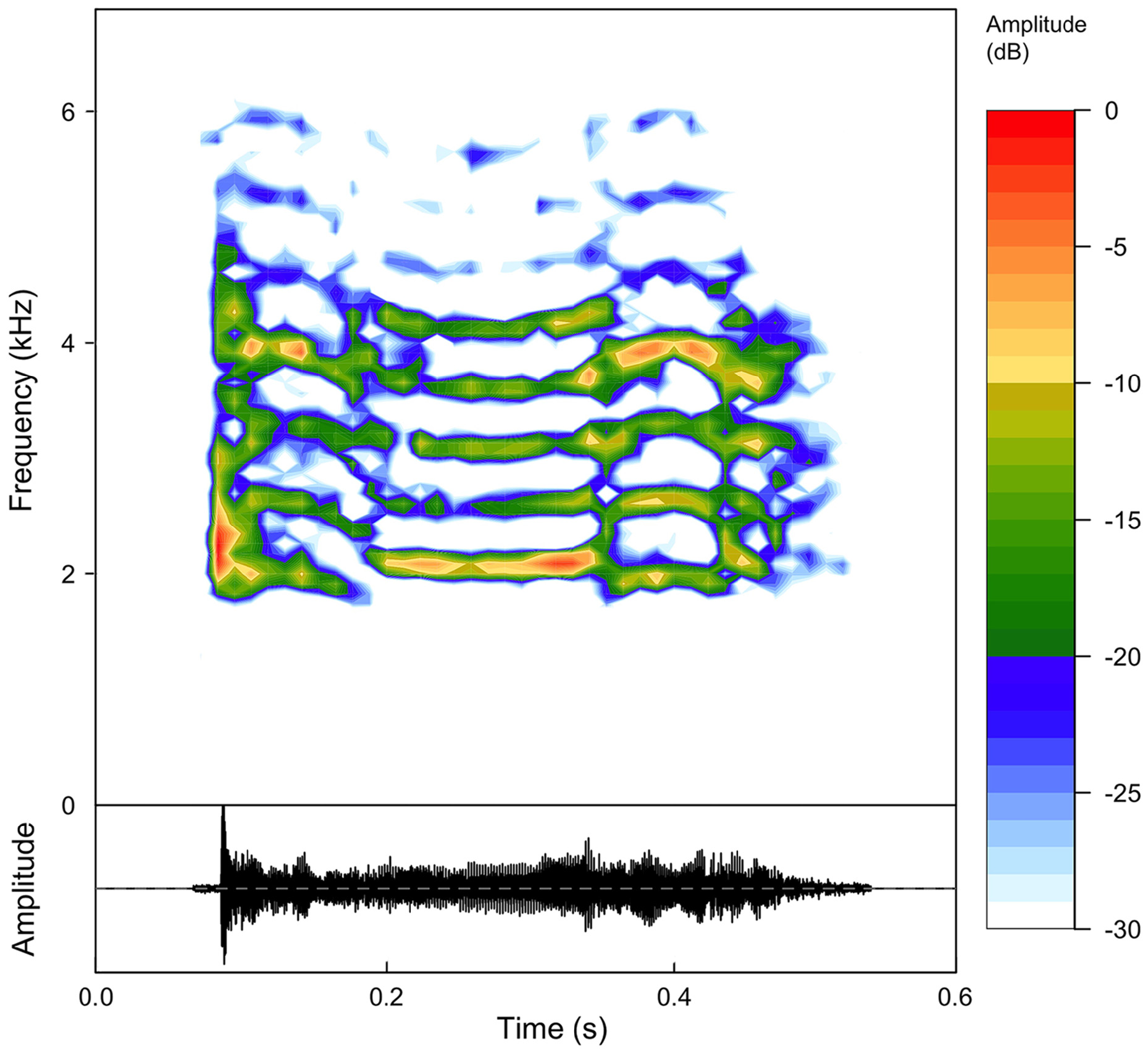
Call. There was no significant variation between the calls of Thoropa bryomantis sp. n. from the type locality and those reported by Nunes-de-Almeida et al. (2016) ( Table 4). Based on our results, combined with those of these authors, the call of the new species is characterized by one short note with up to 10 harmonics ( Fig. 7 View FIGURE 7 ). Call duration ranges 0.23– 0.42 s, with intervals ranging 30.9–241.4 s. The peak of the dominant frequency ranges 2060–4470 Hz, the minimum frequency ranges 1890–2411 Hz, and the maximum frequency ranges 4134–6540 Hz.
Principal component analysis. The first two components extracted from the PCA of morphometric variables explained 81.8% of the variance in the dataset. The most explanatory variables were snout-vent length (SVL), head length (HL) and head width (HW) for PC 1, and eye diameter (ED), foot length (FL) and interorbital distance (IOD) for PC 2 ( Table 2). Plotting PC1 vs. PC2 revealed a distinct grouping of the populations of the new species separate from T. lutzi and partially overlapping with T. petropolitana ( Fig. 8 View FIGURE 8 ).
The first two components extracted from the PCA of acoustic parameters explained 84.2% of the variance in the dataset. The most explanatory variables were minimum and maximum frequency for PC 1, and call duration and peak of the dominant frequency for PC 2 ( Table 3). Plotting PC1 vs. PC2 revealed a clear separation of species according to variables of their advertisement calls and that the populations included in the type-series were again nested within the same acoustic space ( Fig. 9 View FIGURE 9 ).
Habitat and Natural History. We collected Thoropa bryomantis sp. n. in the field from two granitic outcrops, both more than 600 m a.s.l, in the municipalities of Antônio Prado de Minas and Cataguases in the state of Minas Gerais. Although we visited the same areas during every rainy season (October to March), specimens were seen/ heard only on rainy days. Specimens emitted calls mostly in wet places with the presence of bryophytes on rocky walls ( Fig. 5C View FIGURE 5 ). It is worth mentioning that several males were recorded during reproductive activity in areas adjacent to rocky outcrops, including cattle pastures in the municipality of Antônio Prado de Minas. The new species occurs in sympatry with Scinax cosenzai Lacerda, Peixoto & Feio, 2012 (see Neves et al. 2016) and its congener Thoropa miliaris , the latter being observed vocalizing within less three meters from Thoropa bryomantis sp. n.
Geographic distribution. Thoropa bryomantis sp. n. is only known to occur in the northern region of the Serra da Mantiqueira in Southeast Brazil, in the municipalities of Santa Teresa, Muniz Freire and Alegre (state of Espírito Santo) and Alto Caparaó, Antônio Prado de Minas , and Cataguases (state of Minas Gerais) ( Fig. 10 View FIGURE 10 ) .
Etymology. The epithet bryomantis is Greek for moss frog ( bryos = moss; mantis = anuran/frog), in reference to the substrate it occupies and by which it is camouflaged (rocks covered by moss). The name is used as a noun in apposition.
Conservation status. Thoropa lutzi is currently categorized as Data Deficient (DD) in the state of Rio de Janeiro ( Bergallo et al. 2000) as well as by the national list of endangered species (ICMBio 2014), and as Endangered (EN) by the IUCN Red List ( Carvalho-e-Silva & Carnaval 2004). However, after comparing our recently collected specimens to those historically identified as T. lutzi (see Bokermann 1965, Cocroft & Heyer 1988, Izecksohn & Carvalho-e-Silva 2001, Carvalho-e-Silva & Carnaval 2004, Gasparini et al. 2007, Almeida et al. 2011, Haddad et al. 2013, Nunes-de-Almeida et al. 2016), we conclude that individuals from the states of Espírito Santo and Minas Gerais should be identified as T. bryomantis sp. n. Unfortunately, the occurrence of T. lutzi in the municipality of Mimoso do Sul (ES), reported by Almeida et al. (2011), has no voucher, so we could not confirm its species identification. Our results indicate that T. lutzi should be considered endemic to the state of Rio de Janeiro. Furthermore, it is worth mentioning that the species has not been found in nature in the last 42 years (last record in 1979). Thus, given its geographical restriction and rarity, we suggest that the conservation status of T. lutzi be reviewed.
On the other hand, Thoropa bryomanthis sp. n. has been reported for six municipalities from two different states (ES and MG), including protected areas (e.g., Parque Nacional do Caparaó), and so does not seem to be threatened with extinction. Furthermore, the populations recorded in the state of Minas Gerais seem to be abundant, with calling activity taking place even in anthropized areas such as cattle pastures.
Remarks. Although the presence of a vocal sac and vocal slits has been reported for Thoropa miliaris and T. taophora ( Feio et al. 2006) , we did not observe these structures in these species, nor in any of the other larger-sized species ( T. megatympanum and T. saxatilis ). There are differences in temporal, spectral and structural parameters between the calls of the large and small-sized species of Thoropa ( Nunes-de-Almeida et al. 2016). The advertisement call of the small-sized species ( Thoropa bryomanthis sp. n., T. lutzi and T. petropolitana ) have a structure similar to that of anuran defensive screams, and to calls of anuran species that vocalize with their mouths open (e.g., Toledo et al. 2009, Grid-Pap 2008). These acoustic similarities led to the hypothesis that the small-sized species of Thoropa also open their mouths wile vocalizing, which would explain the acoustic differences between the two phenetic groups of Thoropa ( Nunes-de-Almeida et al. 2016). However, our observations do not support this hypothesis since Thoropa bryomanthis sp. n. vocalizes with the mouth closed ( Fig. 6D View FIGURE 6 ). Considering that vocal sacs and slits influence the sounds produced by anurans ( Pauly et al. 2006), these acoustic differences are probably related to the presence and absence of these structures in the small and large-sized species of Thoropa , respectively. In addition, the presence of a vocal sac and vocal slits in the new species raises doubt about the position of the small-sized species within the genus and reinforces the hypothesis of a paraphyletic Thoropa ( Sabbag et al. 2018) .
breviations of Brazilian states: MG = Minas Gerais ; RJ = Rio de Janeiro.
Parameter Thoropa bryomantys sp. n. T. lutzi T. petropolitana
Call duration (s) 0.26–0.42 0.23–0.31 0.18–0.20 0.034 –0.037 (0.32±0.05) (0.27±0.02) (0.19±0.005) (0.036±0.001)
Interval between calls (s) 30.9–49.7 40.5–241.4 9.4–46 14.6–31.5 (36.8±6.8) (85±56.2) (23.7±11.7) (23.3±5.8)
Peak of dominant frequency (Hz) 2067–2584 2060–4470 1723–1723 3000–4125 (2440.4±230.3) (2360±430) (1723) (3812±400)
Minimum frequency (Hz) 1894–2411 1890–2060 517–1033 2250–3000 (2210±218) (2000±80) (812±150) (2562±260)
Maximum frequency (Hz) 4134–5684 4300–6540 2928–3100 4500–5625 (4751±384) (4770±570) (2977±70) (5062±420)
Notes per call 1–1 1–1 1–1 1–1
Pulses per note – – – 11–12 (11.5±0.37)
Municipality, state Cataguases, MG Antônio Prado de Rio de Janeiro, RJ Petrópolis, RJ ( type locality) Minas, MG ( type locality) ( type locality)
Number of calls analyzed and 12 calls; 3 males 30 calls; 2 males 8 calls; 1 male 6 calls; 1 male males recorded
Reference This study Nunes-de-Almeida Nunes-de-Almeida Nunes-de-Almeida et al. (2016) et al. (2016) et al. (2016)
| MG |
Museum of Zoology |
| R |
Departamento de Geologia, Universidad de Chile |
| T |
Tavera, Department of Geology and Geophysics |
| V |
Royal British Columbia Museum - Herbarium |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Thoropa bryomantis
| Assis, Clodoaldo Lopes, Lacerda, João Victor A., Guimarães, Carla Silva, Peixoto, Marco Antônio, Luna, Maria Celeste & Feio, Renato Neves 2021 |
Thoropa aff. lutzi
| Ferreira, R. B. & Monico, A. T. & Silva, E. T. & Lirio, F. C. F. & Zocca, C. & Mageski, M. M. & Tonini, J. F. R. & Beard, K. H. & Duca, C. & Soares, T. 2019: 143 |
Thoropa cf. lutzi
| Sabbag, A. F. & Lyra, M. L. & Zamudio, K. R. & Haddad, C. F. B. & Feio, R. N. & Leite, F. S. F. & Gasparini, J. L. & Brasileiro, C. A. 2018: 147 |
Thoropa lutzi
| Sabbag, A. F. & Lyra, M. L. & Zamudio, K. R. & Haddad, C. F. B. & Feio, R. N. & Leite, F. S. F. & Gasparini, J. L. & Brasileiro, C. A. 2018: 143 |
| Nunes-de-Almeida, C. H. L. & Assis, C. L. & Feio, R. N. & Toledo, L. F. 2016: 1 |
| Haddad, C. F. B. & Toledo, L. F. & Prado, C. P. A. & Loebmann, D. & Gasparini, J. L. & Sazima, I. 2013: 175 |
| Almeida, A. P. & Gasparini, J. L. & Peloso, P. L. V. 2011: 544 |
| Gasparini, J. L. & Almeida, A. P. & Cruz, C. A. G. & Feio, R. N. 2007: 76 |
| Carvalho-e-Silva, S. P. & Carnaval, A. C. 2004: 1 |
| Izecksohn, E. & Carvalho-e-Silva, S. P. 2001: 88 |
| Cocroft, R. B. & Heyer, W. R. 1988: 216 |
| Bokermann, W. C. A. 1965: 526 |