Monodelphis glirina (J. A. Wagner, 1842)
|
publication ID |
https://doi.org/10.11646/zootaxa.4508.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:390204FC-FE44-4D16-9F06-6443C4460166 |
|
DOI |
https://doi.org/10.5281/zenodo.3717671 |
|
persistent identifier |
https://treatment.plazi.org/id/DC558798-FF9F-FFCA-FF5B-FDDAFEB1B57D |
|
treatment provided by |
Plazi |
|
scientific name |
Monodelphis glirina |
| status |
|
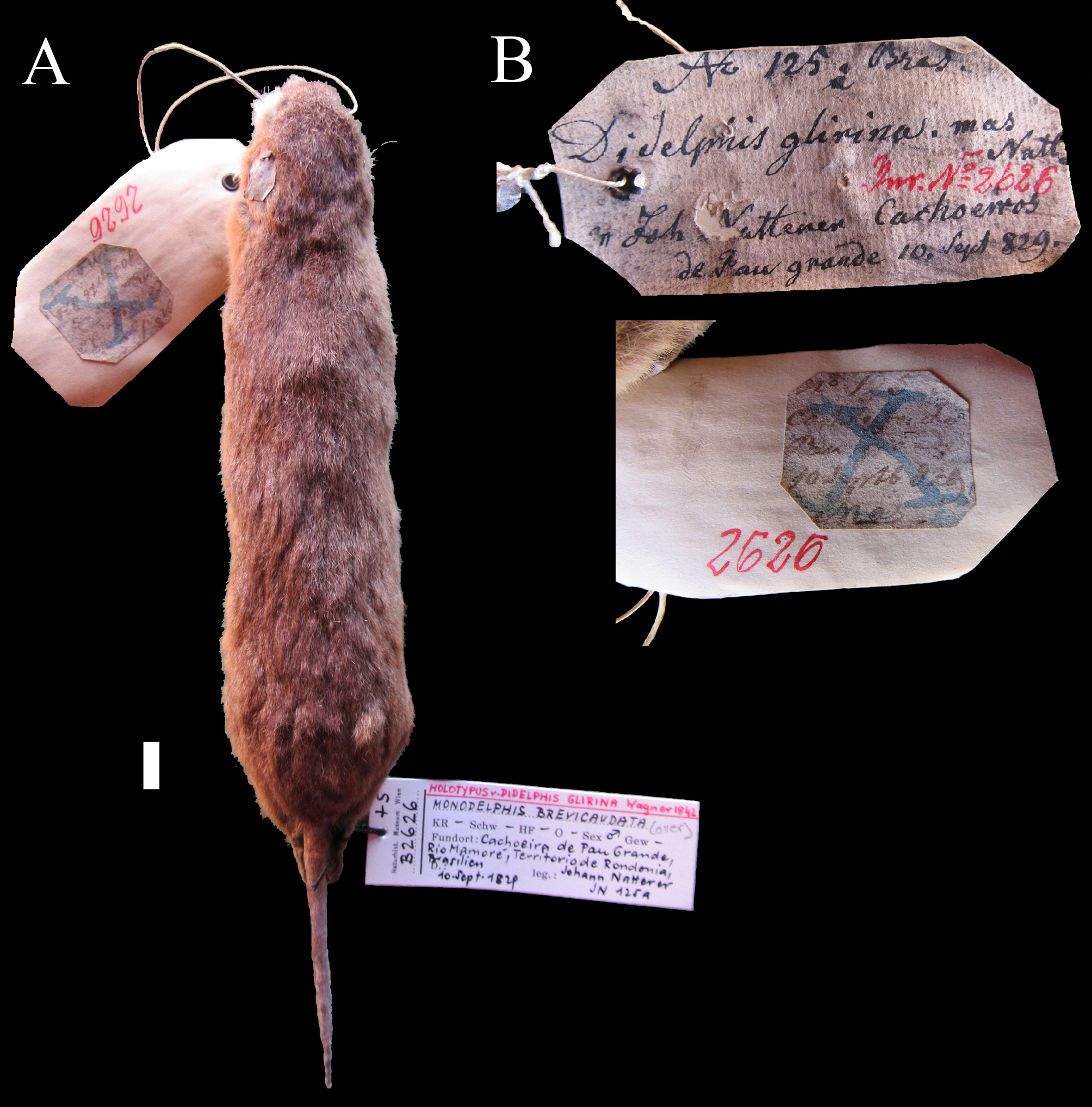
Type specimen of Monodelphis glirina View in CoL .
Natterer collected a single Monodelphis specimen, NMW B 2626 , in Mamoré , Rondônia state, at Cachoeira do Pau Grande, on the September10 th, 1829 ( Fig. 2 View FIGURE 2 ), referred as the holotype in some publications (e.g., Pine et al. 2013; Pavan & Voss 2016). The September 1829 date is present in Pelzeln’s (1883) monograph on mammal specimens collected by Natterer in Brazil and this is the only Monodelphis specimen known from that locality. Presently, this specimen has pelage coloration, much of which is depigmented, showing an almost homogeneous faint grey ochre grizzled dorsal coloration, with lighter patches along the pre and post auricular region, and the ventral side is pale orange with base light-gray and pale orange tip ( Fig. 2 View FIGURE 2 ).
Geographic Distribution. A new locality (loc. 2 in Fig. 1 View FIGURE 1 ) herein reported extends the presently known species range by more than 350 km from the known southeastern limit (loc. 12 in Fig. 1 View FIGURE 1 ). Habitat descriptions from the sampled localities and the vouchers obtained in each area are provided below:
1) Humaitá municipality [locality 1 in Fig. 1 View FIGURE 1 ], southern Amazonas state (AM), Brazil: one adult specimen (UNB 2041, field number ARB 310) was sampled during an inventory carried out in the dry season, between July 17 and August 0 3, 2003, at the 54° ‘Batalhão de Infantaria de Selva’ (a military area of Brazil, located at 7° 31' S, 63° 02' W, ca. 60 m altitude). In this municipality, the landscape comprises islands of savanna vegetation embedded within a typical Amazon forest matrix ( Pires 1973). Sampling effort comprised 1,920 trap-nights, using 20 Sherman® traps in latosol open grassland savanna habitat in transition to open rainforest, and with 100 pitfall traps disposed in a latosol tree savanna and adjacent rainforest (IBGE 2004). The specimen was captured in a Sherman® trap at the border of open savanna with semideciduous forest.
2) Confresa municipality [locality 2 in Fig. 1 View FIGURE 1 ], northeastern Mato Grosso state (MT), Brazil: five specimens were collected during an inventory carried out during the dry season in May 2006 at Fazenda da Destilaria Gameleira (a local farm), ( 10° 32' 21.5"S, 51° 23' 21.5" W, ca. 60 m altitude), including two females (UNB 4074/ ARB 693 and UNB 4075 ARB 694) and one male (UNB 4071/ARB 687) captured in Sherman® traps, while two individuals (female UNB 4072/ARB 688 and male UNB 4073/ARB 691) were captured in pitfall traps. All specimens were captured in a landscape composed of Amazon forest with semideciduous forest under intense agricultural activities (IBGE 2004). Shermans® and pitfalls traps were placed as described above, but the capture effort was 405 trap-nights.
A previous record, Locality 13, included in the sample EF154215 View Materials (field number and voucher number, AN 1007 and MPEG 34899, respectively), was wrongly identified, including the coordinates first used in Carvalho et al. (2011), and after by Pavan et al. (2014). The correct locality is Vilhena, also Rondônia state, Brazil (Appendix A). The locality was here corrected using the voucher catalogue of the MPEG’s mammal scientific collection and coordinates provided in the supplementary materials of Mesquita et al. (2007), as the specimen AN 1007 had been sampled in the same field survey.
Measurements of herein sequenced specimens and those of the holotype of M. glirina are given in Table 1. The specimen from Humaitá, AM (UNB 2041/ARB 310), was almost 50% larger than the subadult individuals from Confresa, MT (UNB 4072/ARB 688, UNB 4074/ARB 693, and UNB 4075/ARB 694) ( Figure 3 View FIGURE 3 ), and three times heavier ( Table 1). Cranial measurements from the Humaitá specimen are also larger, as evidenced by comparing the skulls of these specimens ( Fig. 3 View FIGURE 3 ).
Pelage coloration also varied between the two samples ( Fig. 4 View FIGURE 4 ). The specimen from Humaitá (AM) has orange (Dresden Brown) dorsal coloration on the lateral parts of head, postauricular region, throat and rump, and on the lateral hindlimbs. Middle dorsal line pelage from the nose to the rump is a grizzled light-gray (Deep Grayish Olive), with the base subtly darker than tips. The ventral coloration, with a gradual separation from the dorsal side, is pale orange with fur length in the middle venter ca. 6 mm, base light-gray (Iron Gray) and with pale orange tips (Saccardo’s Olive). The tail is dark-grey (Chaetura Black) above and faintly clearer below, with orange body fur covered the first 15 mm of the tail (ca. 1/5 of the tail length).
Three reproductive subadult females (class 3 by van Nievelt & Smith 2005) from Confresa (MT) (UNB 4072/ ARB 688—with left deciduous dP3 above an erupting P3, right P3 erupting, and erupting M4; UNB 4074/ARB 693—almost functional P3 and M4; and UNB 4075/ARB 694—P3 and M4 erupting) show dorsal coloration from nose to rump uniformly Deep Grayish Olive (near Dark Mouse Gray basally and Sepia distally), and orange-gray on the lateral sides of head and post-auricular region (near Dark Mouse Gray basally and Metal Bronze distally). The ventral coloration, with subtle separation from dorsal side, shows pale gray coloration with fur length in the middle venter ca. 4 mm, base gray (near Dark Quaker Drab) and with pale orange tips (Grayish Olive). The tail is light-gray on both sides, with body fur covering the first 10 mm of the tail of the adult specimen (1/6 of the tail length). Two juvenile males (UNB 4071/ARB 687 and UNB 4073/ARB 691 with deciduous dP3 and erupting M3), class 2 by van Nievelt and Smith (2005), have a more uniformly gray and less grizzled dorsal coloration than the females, and not differing from the lateral sides, except on the face below the eyes and in the preauricular region. The tail is gray (Olivaceous Black 1) in UNB 4073/ARB 691 and grayish brown (Bister) in UNB 4071/ARB 687, without difference between either side.
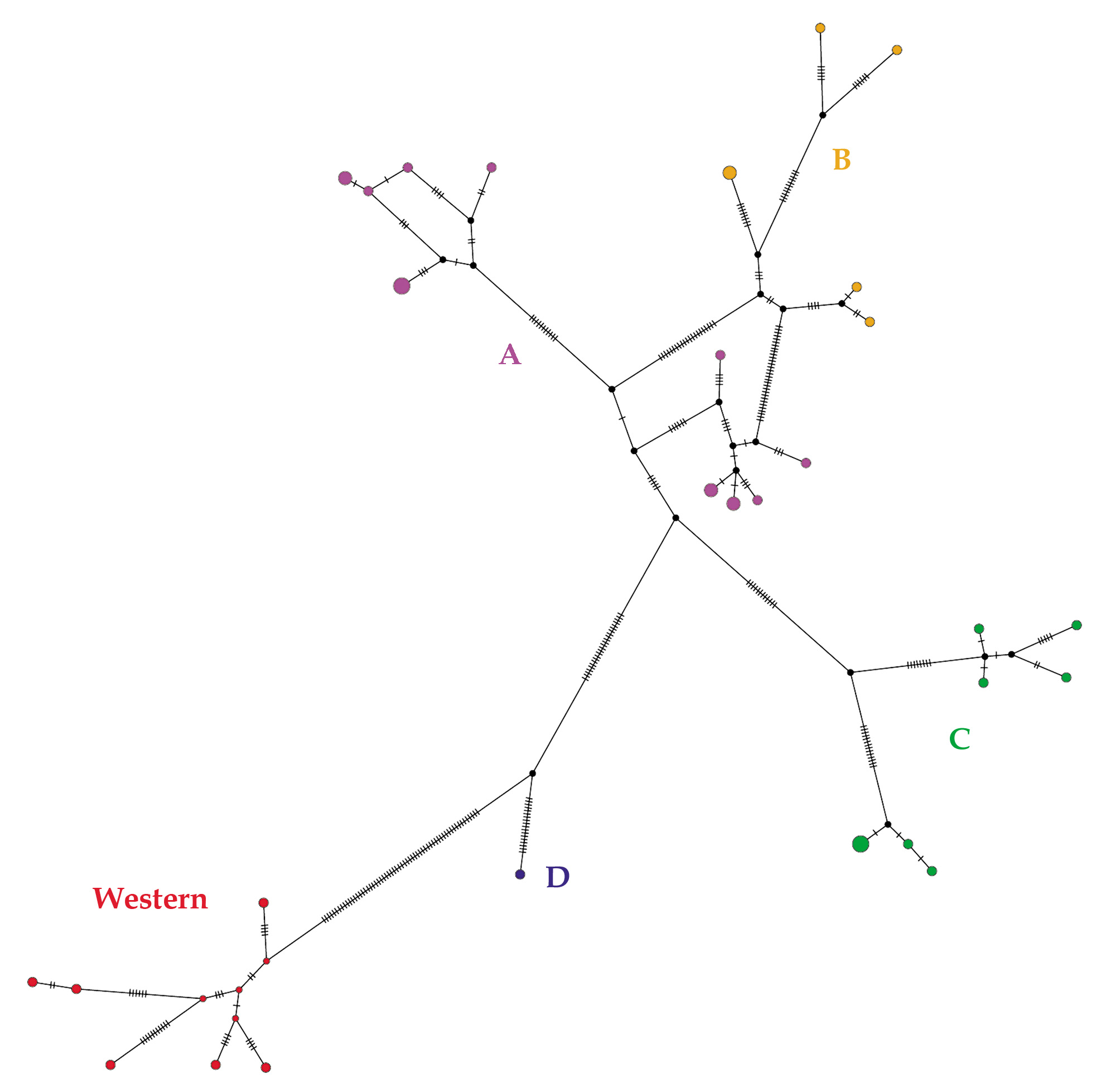
Molecular analyses. Phylogenetic trees built with BI and ML methods produced the same topology ( Fig. 5 View FIGURE 5 ). Two main clades, a “western” clade and an “eastern” clade, were retrieved diverging by an average genetic distance of 8.5 % ( Table 2, Fig. 6 View FIGURE 6 ). The “eastern” clade was only fairly supported and is genetically structured with the presence of four lineages named A, B, C, and D. These have generally low support with BI, with exception of lineage D, but moderately or highly supported with ML, with exception of lineage A.
The TCS network retrieved similar relationships ( Fig. 6 View FIGURE 6 ) as showed by the phylogenetic tree. The highest number of substitutions is observed between the “western” clade and the other haplotypes belonging to “eastern” clade. The C and D lineages are clearly separated, while haplotypes belonging to the A and B lineages are linked by lower number of mutational steps.
The four lineages are geographically separated in four different patches ( Fig. 7 View FIGURE 7 ). Genetic distances among lineages were quite high (range 4–6.7%), but within lineages mean distance divergence was low (range 0.7–1.8%) ( Table 2). In contrast, the “western clade is more homogenous (mean distance within clade 1.2 %). New samples from Mato Grosso state fall within the “eastern clade, specifically subclade C, together with individuals from localities 9 and 10 (Appendix B). The sample from Humaitá fell within the “western clade.
In order to assign the sequence KM 071375 View Materials (by Pavan et al. 2014) from a topotype of M. maraxina (BMNH 24.2.4.43), from Caldeirão, Gurup, Maraj archipelago, Par state, Brazil (locality 21), we ran a NJ tree with a reduced alignment (38 sequences, 800 bp) to avoid excessive gaps. The obtained tree unequivocally assigned the specimen to the lineage C of the “eastern clade (97 % bootstrap value) (Appendix C), confirming the results by Pavan et al. (2014).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |