Tenucephalus DeLong
|
publication ID |
https://doi.org/10.11646/zootaxa.4954.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:A8D2AA60-562C-4F98-8000-D792F1E40C87 |
|
DOI |
https://doi.org/10.5281/zenodo.4701134 |
|
persistent identifier |
https://treatment.plazi.org/id/DE47C351-FFC1-C164-FF67-DECAFA6A7EEC |
|
treatment provided by |
Plazi |
|
scientific name |
Tenucephalus DeLong |
| status |
|
Tenucephalus DeLong View in CoL
Type species: Tenucephalus marginellus DeLong, 1944 ; designated.
Tenucephalus DeLong, 1944: 236 View in CoL [original description, new species, morphology, illustration]; Evans, 1947: 141 [classification]; Linnavuori, 1957: 141 [description, illustration]; Metcalf, 1963: 60 [catalogue]; Linnavuori & DeLong, 1976: 29–31 [description, new species, illustration, distribution]; Linnavuori & DeLong, 1977b: 558–559 [new species, illustration]; Cwikla & Blocker, 1981: 172, 176 [morphology]; Hamilton, 2000: 452 [morphology, classification]; Zanol, 2006: 100 [catalogue]; Zahniser & Dietrich, 2010: 496, 498, 508 [phylogeny, morphology, classification, DNA sequences]; Zahniser & Dietrich, 2013: 7, 16–18, 85 [phylogeny, classification, DNA sequences]; Zahniser, 2007 [online catalogue]; Freytag & Gaiani, 2017 [online catalogue]
Diagnosis. Tenucephalus can be differentiated from other genera of the tribe by the macropterous males and females, the dorsum without distinct longitudinal orange stripes, anterior margin of the head with numerous transverse carinae, ocelli close to eyes, discal portion of crown smooth and shiny, lateral margin of pronotum much shorter than greatest width of eye, subgenital plates with macrosetae uniseriate laterally, connective fused to aedeagus, ovipositor extending very far beyond pygofer apex, and first valvula dorsal sculpturing maculose and dorsoventrally elongated.
Body. Male, 3.3–5.5 mm. Female, 3.9–6.2 mm to wing apex, 4.0– 7.1 mm to ovipositor tip. Body length 3.9– 4.2x width of pronotum (males), 4.0–4.9x (females). Anterior margin of head with numerous transverse carinae. Crown length (males and females) 0.5x–0.7x interocular width; texture smooth, shiny, with faint longitudinal striae; with transverse depression before carinae on anterior margin. Ocelli very close to eyes; on anterior margin. Frontoclypeus texture shagreen; convex. Antennal sockets situated near middle of eye. Pronotum carinate laterally; texture smooth, shiny, with shallow transverse furrows. Scutellum texture shagreen to rugose. Protrochanter without distinct setae. Profemur row AV without setae or with short fine setae basally, apically with 3–5 stout closely spaced setae before intercalary row; intercalary row with 6–9 long fine setae; AV1 present; AM1 present; dorsally with pair of apical macrosetae. Protibia macrosetae 1+4 or 1+5. Mesofemur row AV with numerous stout closely spaced setae. Mesotibia dorsal macrosetae 4+4, 4+5, 5+4, or 5+5. Metafemur apical setae 2+2+1. Metatibia arched throughout length. Metatarsomere I long, slender, longer than II +III combined; apex with row of 3 platellae flanked on each side by tapered seta. Macropterous.
Color. Ground color whitish, yellow, tawny, or brown. Crown and pronotum sometimes marked with orange. Anterior margin of head sometimes with transverse white band bordered by thinner dark brown band above and below. Forewings unmarked or with brownish markings outlining wing veins.
Male. Pygofer incised dorsally nearly halfway to base or closer to base; with or without dorsal pair of processes; without ventral process; with moderate to extensive long macrosetae; sometimes with distinct patches of smaller setae. Subgenital plates usually long, triangular, sometimes shortened, subquadrate; usually with uniseriate row of macrosetae and with numerous long fine setae, sometimes with more than one row of macrosetae laterally. Valve triangular. Connective and aedeagus fused (referred to here as “connective-aedeagus”). Connective-aedeagus with anterior arms of connective elongate, narrow, closely appressed; with or without long or short symmetrical pair of processes fused near aedeagal base; with or without asymmetrical unpaired dorsal process articulated or fused near base of aedeagus; aedeagus shape variable, symmetrical or asymmetrical; aedeagus shaft arising ventrally from base of connection with connective; with or without apical processes or flanges. Style broadly bilobed at base; apophysis shape variable. Phragma forming pair of setose bulbous lobes articulating with dorsal rim of base of aedeagus. Segment X sclerotized or membranous; if sclerotized, relatively long, tubular.
Female. Pygofer long; with numerous long macrosetae. Ovipositor elongate; extending very far beyond pygofer apex. Sternite VII shape variable. First valvula slender, straight; dorsal sculpturing maculose with maculae dorsoventrally elongated, submarginal with broad unsculptured band along dorsal margin; VSA present, distinctly delimited, sculpturing similar to dorsal sculpturing. Second valvula lanceolate, narrow; without dorsal teeth. Gonoplac with distinct row of macrosetae.
Distribution. This genus is known from southern Mexico to northeastern Argentina.
Remarks. The male genitalia of Tenucephalus spp. are particularly diverse, but all species share the connective fused to the aedeagus. Most species are known from only one locality, suggesting that further collecting in other areas will yield many undescribed species.
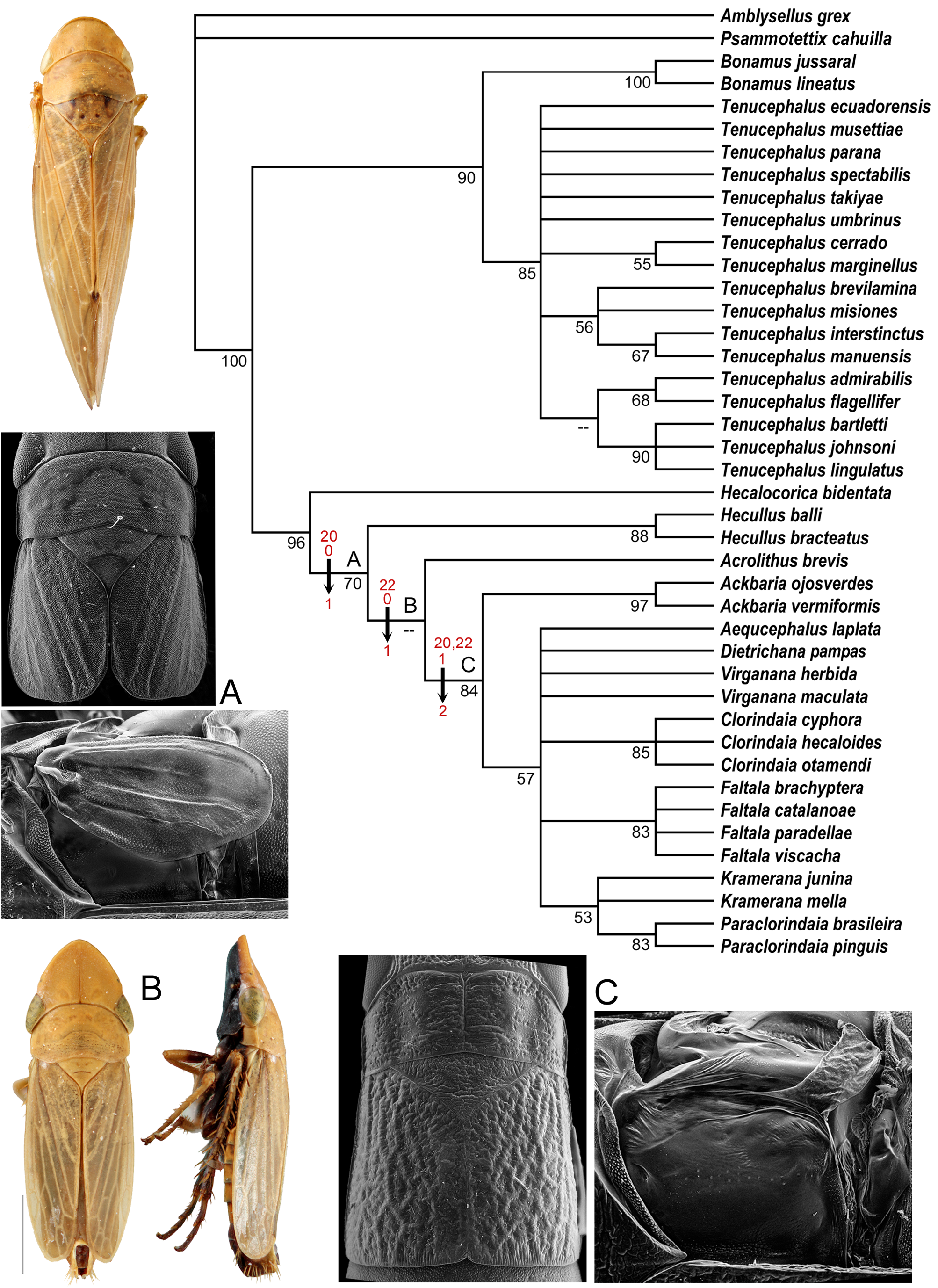
Two general color forms were distinguished here. The “typical color pattern” is a general yellowish to tawny color, anterior margin of the head with a transverse white band bordered above and below by a thin dark brown or black line, and forewing without or with varying amounts of brown shading along wing veins but not forming distinct speckling and spots as in the – interstinctus color pattern. The – interstinctus color pattern (present in T. amabilis , T. heppneri , T. interstinctus , T. misiones , T. manuensis , and T. brevilamina ) is a general background color of whitish or ivory to tawny, anterior margin of the head with or without transverse white band but if present then not completely bordered both above and below by thin dark brown or black line, pronotum with three faint orange longitudinal lines, and forewing with many cells with bases and apices dark brown, forming an overall speckled pattern. The four species included in the phylogenetic analyses here that possess the – interstinctus pattern were resolved as a monophyletic group, but with low branch support ( Fig. 91 View FIGUIRE 91 ).
Seventeen species of Tenucephalus were included in the phylogenetic analyses here. The genus was strongly supported as a monophyletic group and strongly supported in a sister-group relationship to Bonamus . In addition to recovery of the – interstinctus group mentioned above, some relationships among species were supported by the characters included here, but relationships among others were unresolved. The – lingulatus group as referred to below is united by the connective-aedeagus with a distinct single asymmetrical process arising from near the base of the aedeagus and extending dorsad of aedeagal shaft and comprises T. admirabilis , T. bartletti , T. flagellifer , T. johnsoni , and T. lingulatus . Both the – interstinctus and – lingulatus groups are considered informal species groups.
The known geographic range of Tenucephalus is greatly expanded here. Its prior distribution stretched from southern Mexico to Peru ( T. sagittarius ) and northwestern Bolivia ( T. quadricornis ). The twelve new species described from southeastern Brazil and the northeastern panhandle of Argentina ( Fig. 85 View FIGUIRE 85 ) expand the known distribution across the South American continent. Many other localities and new species are newly documented here in southeastern Peru, Brazil (Amazonas), Bolivia, and Ecuador ( Fig. 84 View FIGUIRE 84 ). Localities of most species indicate that they are forest-dwelling, although several have been collected in open habitats.
Included species:
admirabilis n. sp. ( Argentina)
amabilis Linnavuori & Heller, 1961 n.comb. ( Peru)
bartletti n. sp. ( Peru)
brevilamina n. sp. ( Argentina)
cerrado n. sp. ( Brazil)
ecuadorensis n. sp. ( Ecuador)
flagellifer n. sp. ( Peru)
heppneri n. sp. ( Peru)
iguazu n. sp. ( Argentina)
interstinctus n. sp. ( Peru)
johnsoni n. sp. ( Brazil)
lingulatus n. sp. ( Bolivia, Peru)
longicauda Linnavuori & DeLong, 1977b ( Panama)
manuensis n. sp. ( Peru)
marginellus DeLong, 1944 ( Mexico)
misiones n. sp. ( Argentina)
musettiae n. sp. ( Brazil)
nielsoni n. sp. ( Brazil)
novafriburgo n. sp. ( Brazil)
parana n. sp. ( Argentina)
pusillus Linnavuori & DeLong, 1978 ( Panama)
quadricornis Linnavuori & DeLong, 1976 ( Bolivia)
sagittarius Linnavuori & DeLong, 1976 ( Peru)
spectabilis n. sp. ( Peru)
takiyae n. sp. ( Brazil, Peru)
umbrinus n. sp. ( Brazil)
viperinus n. sp. ( Argentina)
Key to species (males) of Tenucephalus
( T. amabilis & T. pusillus not included, known only from female)
1. Connective-aedeagus without a distinct asymmetrical unpaired dorsal process extending dorsad of aedeagal shaft and without a symmetrical pair of processes fused near aedeagal base (76G,H)............................................. 2
1’. Connective-aedeagus with either a distinct asymmetrical unpaired dorsal process extending dorsad of aedeagal shaft (60G,H) or with a symmetrical pair of processes fused near aedeagal base (80G,H)...................................... 6
2(1’). Aedeagus with pair of apical processes (83G,H); aedeagal shaft strongly sinuate and asymmetrical in ventral or caudal view (83H)..................................................................................... T. viperinus View in CoL
2’. Aedeagus without pair of apical processes; aedeagal shaft not strongly sinuate, symmetrical or only slightly asymmetrical in ventral or caudal view................................................................................ 3
3(2’). Pygofer in lateral view strongly depressed, long, narrow, and tapering to apex; pygofer with dorsal processes arising from near base, extending length of pygofer, upturned at apex (74D)........................................... T. musettiae View in CoL
3’. Pygofer in lateral view not strongly depressed; pygofer dorsal processes not matching description above.............. 4
4(3’). Pygofer with weakly sclerotized dorsal processes (66D); subgenital plate short, concave laterally, with 5–6 long macrosetae laterally (66F); aedeagus long, only slightly bent in lateral view, twisted asymmetrically (66G,H).............. T. iguazu View in CoL
4’. Pygofer with strongly sclerotized dorsal processes (76D); subgenital plate longer, not or only slightly concave laterally, with 10 or more long macrosetae laterally (76F); aedeagus strongly curved in lateral view (76H)......................... 5
5(4’). Dorsal pygofer processes more or less paralleling dorsal margin of pygofer, reaching pygofer apex (82D); aedeagus sickleshaped in lateral view, very narrow in ventral or caudal view (82H,I)................................... T. umbrinus View in CoL
5’. Dorsal pygofer processes directed ventrally, reaching ventral margin of pygofer (76D); aedeagus U-shaped in lateral view, not very narrow in ventral or caudal view (76H,I).................................................. T. novafriburgo View in CoL
6(1’). Connective-aedeagus with an asymmetrical unpaired dorsal process arising from base of aedeagus and extending dorsad of aedeagal shaft for entire length of shaft or longer (not including any accessory apical process of aedeagus, if present), without symmetrical pair of processes fused near aedeagal base (60G,H).............................................. 7
6’. Connective-aedeagus without an asymmetrical unpaired dorsal process arising from base of aedeagus and extending dorsad of aedeagal shaft (two species with symmetrical unpaired process differing than that referred to above— T. takiyae View in CoL with process dorsad of shaft; T. quadricornis View in CoL with process ventrad of shaft), with symmetrical pair of processes fused near aedeagal base (80G,H).......................................................................................... 11
7. Pygofer distinctly constricted in dorsal view, constriction forming a wide base and narrow apical part (60E); aedeagus without apical flagellar process (69H).......................................................................... 8
7’. Pygofer not distinctly constricted in dorsal view (59E); aedeagus with a short (59H,R) or long apical (64H,I) flagellar process............................................................................................. 10
8(7). Valve longer than wide (60F); subgenital plates broad, broadly rounded at apex (60F); style apophysis long, as a long as basal part (base to preapical lobe) (60G)............................................................... T. bartletti View in CoL
8’. Valve wider than long (68F); subgenital plates long, narrow, apex more narrowly pointed (68F); style apophysis short (68F) .................................................................................................. 9
9(8’). Valve produced anteromedially (68F); aedeagal shaft lanceolate, reaching to near midlength of dorsal process (68G,H).............................................................................................. T. johnsoni View in CoL
9’. Valve not or only slightly produced anteromedially (69F); aedeagal shaft tubular, reaching 2/3 length of dorsal process (69G,H).................................................................................. T. lingulatus View in CoL
10(7’). Styles bilaterally symmetrical (64G); style apophysis short, truncate (64G); apical flagellar process of aedeagus very long, extending to apex of dorsal aedeagal process and recurved back to base of aedeagus (64H,I); dorsal process of connectiveaedeagus lanceolate (64H)................................................................... T. flagellifer View in CoL
10’. Styles bilaterally asymmetrical (59G); style apophysis long, not truncate; apical flagellar process of aedeagus short, length only 1.5–2x width of aedeagus at opening of gonopore (59H,R); dorsal process of connective-aedeagus not lanceolate, expanded apically, with two small teeth at ~2/3 length, with preapical long thorn-like process, and apex sharply pointed and constricted preapically (59G,H,Q)............................................................ T. admirabilis View in CoL
11(6’). Paired symmetrical processes of connective-aedeagus short, not reaching midlength of aedeagus (65G,H)............ 12
11’. Paired symmetrical processes of connective-aedeagus long, reaching well beyond midlength of aedeagus (80G,H)..... 15
12(11). With the – interstinctus View in CoL color pattern (70A1,A2,B1,B2); aedeagus with two pairs of apical or preapical processes (70G,H).. ................................................................................................. 13
12’. With the typical color pattern (71A,B); aedeagus without apical processes..................................... 14
13(12). Pygofer with apical thorn-like process; apical aedeagal processes directed dorsally (70D,E); ventral pair of apical aedeagal processes with preapical tooth (70G,H)......................................................... T. manuensis View in CoL
13’. Pygofer without apical process (65D); apical aedeagal processes directed caudally (65H); ventral pair of apical aedeagal processes without preapical tooth (65G,H)........................................................ T. heppneri View in CoL
14(12’). Pygofer process with distinct medial tooth near midlength of process, with minute marginal teeth at apex; subgenital plates short, broadly rounded at apex; style apophysis apex widened apically, not foot-like; aedeagus in lateral view very narrow, long, and evenly curved throughout length.................................................... T. marginellus View in CoL
14’. Pygofer process without distinct medial tooth and without marginal teeth at apex; subgenital plates longer, narrowly rounded at apex; style apophysis apex foot-like; aedeagus in lateral view broader, abruptly angled preapically near gonopore.............................................................................................. T. longicauda View in CoL
15(11’). Connective-aedeagus with additional symmetrical unpaired process arising from near base of aedeagus shaft (80H), emerging either dorsad (80H) or ventrad of shaft base.............................................................. 16
15’. Connective-aedeagus without additional symmetrical unpaired process........................................ 17
16(15’). Pygofer dorsal process expanded toward apex, apex truncate with sharp corners; aedeagus short and broad in lateral view, with accessory dorsal thorn-like process; symmetrical unpaired process of connective-aedeagus originating ventrad of shaft....................................................................................... T. quadricornis View in CoL
16’. Pygofer dorsal process tapered toward apex (80D,E); aedeagus very long and narrow in lateral view (80H), without accessory processes on shaft; symmetrical unpaired process of connective-aedeagus originating dorsad of shaft (80H)..... T. takiyae View in CoL
17(15’). Crown sharply angled to face (79B); crown long, median length 1.5–2x next to eye (79A1,A2); crown largely dark brown (79A1,A2); forewings veins bright yellow, completely bordered by dark brown (79A1,A2,B); subgenital plates with numerous macrosetae loosely arranged laterally in 2–3 rows (79F); aedeagus lying far above pair of basal processes of connectiveaedeagus (79H)............................................................................ T. spectabilis View in CoL
17’. Crown not sharply angled to face; crown shorter; crown without significant dark brown coloration; forewings, if with brown coloration, not as extensive; subgenital plates with at most 12 lateral macrosetae in a uniseriate lateral row; aedeagus not situated high above basal pair of processes of connective-aedeagus.............................................. 18
18(17’). Pygofer without dorsal or apical process (72D,E)......................................................... 19
18’. Pygofer with strongly sclerotized dorsal or apical process................................................... 20
19(18). Subgenital plate very short (61F); style apophysis relatively thick, digitate, with substantial tuberculate texture, not turned laterally at apex, apex blunt (61G); apices of basal paired processes of connective-aedeagus slender, tapered to pointed apex (61G,H)................................................................................. T. brevilamina View in CoL
19’. Subgenital plate not very short (72F); style apophysis with less surface sculpturing, turned laterally at apex, apex pointed (72G); apices of basal paired processes of connective-aedeagus truncate, with pointed tips directed laterally (72G,H)............................................................................................... T. misiones View in CoL
20(18’). Apical pygofer process short, claw-like; aedeagus with accessory pair of preapical falcate processes, arising from ventral side near gonopore............................................................................. T. sagittarius View in CoL
20’. Pygofer process longer, not short and claw-like; aedeagus without accessory apical processes...................... 21
21(20’). Forewing with numerous brown spots, especially at bases and apices of cells (67A1,A2,B); pygofer process arising from inner margin near apex, extending beyond pygofer apex, sinuate, with serrated inner margin and apex (72D,E); aedeagus with very long pair of apical processes (67G,H), processes serrated on dorsal margin (67H)...................... T. interstinctus View in CoL
21’. Forewing without distinct brown spots, although sometimes with brown shading along veins (63A,B); pygofer process not extending beyond pygofer apex, not serrate (63D,E); aedeagus without apical pair of processes (63H,Q)............. 22
22(21’). Style apophysis thick, foot-like, apex pointed and directed laterally (63G); aedeagus robust, dorsal surface roughened by numerous minute teeth inclined anteriorly (63H)................................................ T. ecuadorensis View in CoL
22’. Style apophysis not very thick, not foot-like (62G); aedeagus very slender, without numerous minute teeth on dorsal surface (62H)............................................................................................ 23
23(22’). Pygofer process slender, relatively straight, reaching pygofer apex (62D,E); basal paired processes of connective-aedeagus reaching to midlength of aedeagus or slightly beyond (62G,H); aedeagus with small preapical tooth on dorsal margin (62H)........................................................................................... T. cerrado View in CoL
23’. Pygofer process thicker, turned downward from dorsal margin of pygofer toward ventral margin, not reaching pygofer apex (75D,E); basal paired processes of connective-aedeagus longer, reaching well beyond midlength of aedeagus (75G,H); aedeagus without preapical dorsal tooth (75H)............................................................. 24
24(23’). Pygofer longer (75D,E); style median arm produced anteriorly, extending well anterad of lateral basal lobe (75G); style apophysis ventrally with many fine setae (75G); basal paired processes of connective-aedeagus nearly straight, tapered to apex (75G,H)............................................................................... T. nielsoni View in CoL
24’. Pygofer shorter; style median arm not produced, even with lateral basal lobe (77D,E); style apophysis without fine setae (77G); basal paired processes of the connective-aedeagus slightly curved medially, slightly flared at apex...... T. parana View in CoL
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Deltocephalinae |
|
Tribe |
Faltalini |
Tenucephalus DeLong
| Zahniser, James N. 2021 |
Tenucephalus
| Zahniser, J. N. & Dietrich, C. H. 2013: 7 |
| Zahniser, J. N. & Dietrich, C. H. 2010: 496 |
| Zanol, K. M. R. 2006: 100 |
| Hamilton, K. G. A. 2000: 452 |
| Cwikla, P. S. & Blocker, H. D. 1981: 172 |
| Linnavuori, R. E. & DeLong, D. M. 1977: 558 |
| Linnavuori, R. E. & DeLong, D. M. 1976: 29 |
| Metcalf, Z. P. 1963: 60 |
| Linnavuori, R. 1957: 141 |
| Evans, J. W. 1947: 141 |
| DeLong, D. M. 1944: 236 |