Callithrix flaviceps (Thomas, 1903)
|
publication ID |
https://doi.org/10.5281/zenodo.5730714 |
|
DOI |
https://doi.org/10.5281/zenodo.5730838 |
|
persistent identifier |
https://treatment.plazi.org/id/DF668780-FFDE-FFC9-FA0E-F82D6602E7EB |
|
treatment provided by |
Conny |
|
scientific name |
Callithrix flaviceps |
| status |
|
19 View On .
Bufty-headed Marmoset
Callithrix flaviceps View in CoL
French: Ouistiti a téte jaune / German: Gelbkopf-Blschelaffchen / Spanish: Titi de cabeza amarilla
Taxonomy. Hapale flaviceps Thomas, 1903 ,
Brazil, Engenheiro Rive, municipality of Alegre, SW Espirito Santo (c.20° S, 41° W).
This species hybridizes with C. aurita in south-eastern Minas Gerais State and with C. geoffroyi in Espirito Santo State. C. flaviceps 1s considered by A. F. Coimbra-Filho to be a subspecies of C. aurita . Monotypic.
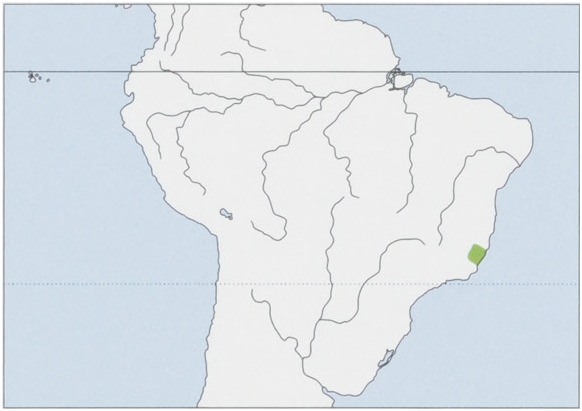
Distribution. SE Brazil in the Serra da Mantiqueira in S Espirito Santo, S of the Rio Doce at least to the state boundary with Rio de Janeiro State, W into E Minas Gerais in the Rio Manhuacu Basin; in the N and W it extends almost to the right bank of the Rio Doce. View Figure
Descriptive notes. Head-body 22-25 cm, tail 30-35 cm; weight c.400 g. The Buffyheaded Marmosetis yellowish gray-brown, with the bases ofits hairs black, speckled buffy upperparts, and a golden-orange underside. Ear tufts are short (c.2-5 cm), fanlike (coming from inside the ear), and yellowish-white. The forehead and crown are yellow-brown, and the muzzle is white. The tail is ringed.
Habitat. Montane and submontane evergreen and semi-deciduous forest. Buffy-headed Marmosets have a preference for successional forest with dense vegetation and a dense understory, and even young secondary forest (“capoeira”). They are least abundant in tall forest with sparse understories. Forests they occupy are highly seasonal in rainfall and temperature. They have been recorded at elevations of 270-1800 m.
Food and Feeding. Diets of the Buffy-headed Marmoset are composed principally of small animal prey (insects, especially Orthoptera , beetles, caterpillars, spiders, phasmids, small amphibians and reptiles, bats, and bird eggs), fruits, plant exudates (gums and nectar), and fruiting bodies (sporocarps) of fungi. In Caratinga Biological Station ( Minas Gerais), fruits are eaten only seasonally and in small amounts. They made a significant contribution to the diet only in the wet season: 65% of the feeding records in January and 53% in February. Fruits/seeds were eaten almost entirely from species of Allophylus (Sapindaceae) in January and from species of Siparuna (Siparunaceae) in February. Species in both genera are small pioneering trees and were abundant in the marmosets’ home range. In November,fruits contributed 18% of the feeding records, but no more than 5% in other months. This was due to a general lack of fruits, not just those that they would find edible (e.g. small, sweet drupes or berries). Nectar of Mabea fistulifera ( Euphorbiaceae ) was eaten in May during the dry season. This concentration on very few species was also true for gums, which they exploited during the rest of the year. Two species of Fabaceae , Acacia paniculata (a vine) and Anadenanthera peregrina (a tree), were common in their home ranges and typical of secondary growth forest; they contributed more than 90% of the gum feeding records. Regarding use of Acacia paniculata, marmosets traplined droplets and accumulations of gum resulting from insect damage. In contrast, they gouged holes in the hard bark of the trunk and branches of Anadenanthera peregrina but also exploited its gum when there was spontaneous flow after heavy insect attack. Sporocarps of two types of ascomycete bamboo fungus (Mycocitrus sp.) were staples in the diet of a group of Buffy-headed Marmosets studied for twelve months in the Augusto Ruschi Biological Reserve ( Espirito Santo); fungi accounted for 65% of the feeding records during the year (54-70% in any one month). Gum-feeding was accordingly reduced to only 6% ofthe diet and fruit 3-3%, with the remainder being animal prey (26%). In months when fruits were more available, the contribution of gum was correspondingly lower. Gums from more than 26 plant species were eaten by the group at Augusto Ruschi, but they gouged trunks and limbs of only two species of Guapira (Nyctaginaceae) and Sloanea (Elacocarpaceae). For all others species, gum was readily available from damage or tree-boring insects.
Breeding. Female Buffy-headed Marmosets produce twins twice a year. A birth peak occurs in the wet season (November—January) and may also occur in the dry season (July-September), evidently depending on food availability that varies from year to year. Infants are carried by the mother on their first day, but this responsibility is relinquished to helpers on the second day. All group members carry offspring; even older siblings, as young as six months old, may try to help. Group members share food items with infants, which become independent at about three months old. As in other species of marmosets, reproduction is usually restricted to one female in each group; the breeding female inhibits reproduction in female subordinates in the group. Occasionally, however, a second female in the group may breed, and this may continue over several years—believed to be an arrangement between mother and a daughter. Births are separated by several months. In this situation, females carry their infants for at least the first week, possibly to avoid infanticide, particularly by the dominant breeding female. Reproductive success of subordinate females is lower; they have fewer and smallerlitters, and infant survival is poor. Four females in a group of 11-16 individuals studied at the Augusto Ruschi Biological Reserve gave birth over a ten-day period. Pregnancies were believed to have been caused by males from neighboring groups. Of the four births, twins of the dominant breeding female, a singleton born to another female, and one twin of each of the two remaining females survived to the end of the study; the other two infants were victims of infanticide. Observations in the wild showed that infants were carried exclusively by the mother on the first day but by other group members after that, and that females rarely carried their infants after the first week. In a group of eleven adults and subadults and two juveniles, infants were carried by ten individuals. Contributions to infant care were variable, however, with the two adult females and a subadult female contributing very little (less than 2% of the overall effort). Adult males carried infants more than any other group members, especially during the first week, and two of them carried the infants to the same extent as the mother. Carriers become increasingly intolerant as the infants grow and often reject them after the second month. Infant care also includes grooming and huddling with infants, food sharing, and sometimes food calling to attract infants.
Activity patterns. Overall, a group of Buffy-headed Marmosets studied at Caratinga Biological Station was active for a relatively short period each day—about nine hours and 46 minutes per day throughout a 13-month study. The group was active longer in the wet season (ten hours and 21 minutes on average) than in the dry season (nine hours and 14 minutes). Individuals were rarely active until at least 20 minutes after it was fully light and retired to their sleeping tree no later than an hour before dusk. Their reduced activity in the dry season may have been related to cooler temperatures. They may have been saving energy or it may have been because of reduced activity of their prey. They spent about 27% of their time foraging for prey, 26% moving about, 25% resting, 11% feeding on plant foods, and 11% in other activities (social, especially grooming and play). There was some seasonal variation in activity; the group spent more time foraging for insects in the dry season (30%) than in the wet season (23%), traveling a little more in dry season (30% vs. 23% in the wet season), and resting less in the dry season (22% vs. 28% in the wet season). Individuals spent more time in social activities in the wet season (15% of their day vs. 8% in the dry season), perhaps because they could afford to do so with more abundant food and they had infants to care for and play with. The increase in time spent foraging in the dry season was accompanied by a lower rate of prey capture, associated with the loss of leaves in the semi-deciduous forest and reduced abundance of the many folivorous insects they eat. Animal prey of the Buffy-headed Marmoset includes particularly small vertebrates, notably more so than has been recorded for other species of marmosets. Lizards and frogs comprised as much as 49% of prey-feeding records in any one month.
Movements, Home range and Social organization. Ecology and behavior of the Buftyheaded Marmoset was studied in the Caratinga Biological Station (1984-1986), an isolated forest of 880 ha, much of which is secondary growth in various stages of succession. Eight groups monitored there had an average size of 9-1 individuals (range 3-16). Group sizes are smaller in the Augusto Ruschi Biological Reserve at 2-5 individuals (mean 3-4). The Buffy-headed Marmoset forms quite stable groups, with dispersal and formation of new groups by both males and females, sometimes migrating together in twos and threes to join others and form new groups. The home range of one group was 35-5 ha, 87-5% of which overlapped with other groups, but without any territorial interactions beyond the exchange of long calls. Monthly home ranges were 16-26 ha, and daily home ranges were 3-6 ha. Distance traveled each day averaged 1222-5 m (range 650-2670 m) and showed little change between wet and dry seasons. A group of 11-16 Buffy-headed Marmosets was studied for a year in the Augusto Ruschi reserve. Fungal sporocarps were the key food source, replacing gums. Fungi at Augusto Ruschi were restricted to widely dispersed bamboo clumps and were scarce (although more abundant in the wet season). A large home range and exploitation of fungi are also found in Goeldi’s Monkey ( Callimico goeldii ). In the early wet season at Caratinga (October-December), groups of Buffy-headed Marmoset are accompanied by kites (Ictinea plumbea and Leptodon cayanensis), capturing cicadas that are flushed into flight by the marmosets. They typically occur in quite low densities: 7 ind/km? (range 3-7-18 ind/km?) at Augusto Ruschi. A higher localized density of 40 ind/km? occurs at Caratinga (estimated total population of 200-300 individuals in 800 ha).
Status and Conservation. CITES Appendix I. Classified as Endangered on The IUCN Red List. The Buffy-headed Marmoset may, in fact, be the rarest of all species of marmosets, with an estimated population of less than 2500 mature individuals. Forests in its restricted distribution in the Rio Doce Valley are now extremely fragmented by agriculture, cattle ranching, Fucalyptus plantations, urbanization, and mining; more than 95% ofthe forest in original distribution of the Buffy-headed Marmoset has been destroyed. Most of the localities where it still exists are small, isolated forest patches with minimal populations. Surveys in 1990-1991 in the municipalities of Caratinga and Ipanema in the Manhuacu Valley found Buffy-headed Marmosets in only four of 16 forest patches (14-216 ha); hybrids of C. flaviceps x C. aurita were found in a fifth patch. They were found in only eleven of 37 forests fragments (c.2-4499 ha) in the six municipalities of the Manhuacu region in 2002-2003. Introduced (released confiscated) Common Marmosets (Callithrixjacchus), Black-tufted-ear Marmosets ( C. penicillata ), and Geoffroy’s Tufted-ear Marmosets (C. geoffroyi ) were found in eight of these forests fragments, and it is believed that they compete and hybridize with Buffy-headed Marmosets. The Buffy-headed Marmoset occurs in the following protected areas: Augusto Ruschi Biological Reserve, Pedra Azul State Park, Forno Grande State Reserve, and Santa Lucia and Sao Lourenco biological stations in Espirito Santo State; Caparao National Park on the border of Espirito Santo and Minas Gerais; Serra do Brigadeiro State Park, Sagui da Serra Municipal Park, Mata do Sossego Private Reserve, and Caratinga Biological Station in Minas Gerais.
Bibliography. Chiarello (2000, 2003), Coimbra-Filho (1971, 1972, 1984, 1986a, 1990), Coimbra-Filho et al. (1981, 1993), Corréa et al. (2002), Cosenza & de Melo (1998), Diego et al. (1993), Ferrari (1987, 1988, 1990, 19913, 1991b, 1992, 2009a, 2009b), Ferrari & Diego (1992, 1993), Ferrari & Lopes (1990b), Ferrari & Mendes (1991), Ferrari & Strier (1992), Grelle & Cerqueira (2006), Guimaraes (1998a, 1998b), Hanson et al. (2003), Hershkovitz (1977), Hilario & Ferrari (2010a, 2010b, 2010c, 2011), Hirsch (2003), Mendes, C.L.S. & de Melo (2007), Mendes, S.L. (1993, 1997a, 1997b), Mendes, S.L. et al. (2009), Mittermeier, Coimbra-Filho & Constable (1980), Mittermeier, Coimbra-Filho, Constable, Rylands & Valle (1982), Natori (1986), Oliver & Santos (1991), Pinto et al. (1993), Porter & Garber (2010), Rylands & de Faria (1993), Rylands, Coimbra-Filho & Mittermeier (1993, 2009), Rylands, da Fonseca et al. (1996), Stevenson & Rylands (1988), Strier et al. (1999), Tabacow et al. (2005).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Callithrix flaviceps
| Russell A. Mittermeier, Anthony B. Rylands & Don E. Wilson 2013 |
Hapale flaviceps
| Thomas 1903 |