Liriomyza sativae Blanchard
|
publication ID |
https://doi.org/10.11646/zootaxa.2850.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/E077879E-FFCA-7F7D-FF44-FCBBFE8B45F4 |
|
treatment provided by |
Felipe |
|
scientific name |
Liriomyza sativae Blanchard |
| status |
|
Liriomyza sativae Blanchard View in CoL
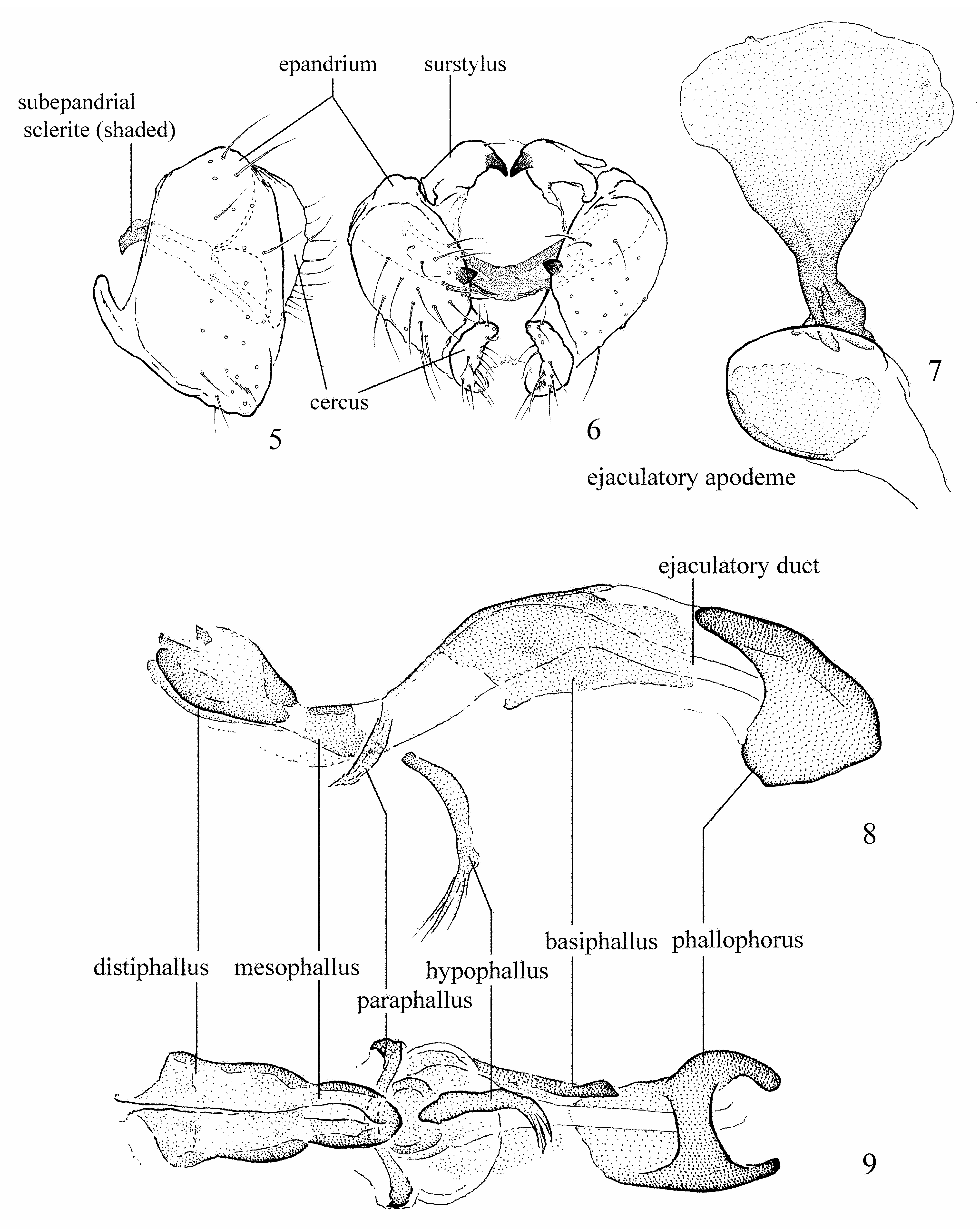
Figs 1 View FIGURES 1–3 , 5–9 View FIGURES 5–9
Liriomyza sativae Blanchard 1938: 354 View in CoL . Frick 1959: 405; Spencer 1973a: 219, 1982: 27, 1983: 59, 1984: 23; Spencer & Steyskal 1986: 292; Rauf et al. 2000: 257; Scheffer & Lewis 2005: 181; Deeming 2006: 410, Palacios et al. 2008: 14 (misidentification, at least in part based on fig. 3).
Liriomyza subpusilla Frost 1943: 255 View in CoL [preoccupied by Malloch, 1914].
Liriomyza verbenicola Hering 1951: 43 View in CoL . Syn. Spencer & Steyskal (1986).
Liriomyza pullata Frick 1952b: 509 View in CoL . Syn. Spencer (1973a).
Liriomyza canomarginis Frick 1952b: 511 View in CoL . Syn. Spencer (1973a).
Liriomyza minutiseta Frick 1952b: 512 View in CoL . Syn. Spencer (1973a).
Liriomyza propepusilla Frost 1954: 73 View in CoL [replacement name for subpusilla View in CoL ]. Frick 1957: 62. Syn. Steyskal (1973).
Liriomyza munda Frick 1957: 61 View in CoL . Syn. Spencer (1973a).
Liriomyza pictella (Thompson) View in CoL . Misidentification, in part. Frick 1957: 66.
Liriomyza guytona Freeman 1958: 344 View in CoL . Syn. Steyskal (1964) [as syn. L. munda View in CoL ].
Fig. 1 View FIGURES 1–3 . Wing length 1.3–1.6mm (rarely 1.1–1.2mm) ( ♂), 1.4–1.8mm ( ♀). Length of ultimate section of vein CuA 1 divided by penultimate section: 1.6–4.0; vein dm-cu sometimes absent if wing length 1.1–1.3mm (Riverside Co., Deep Canyon). Eye height divided by gena height: 4.4–5.8. Scutum shining to subshining.
Chaetotaxy: Two ori (anterior bristle sometimes reduced to absent), sometimes three; two ors. Acrostichal setulae in four irregular rows.
Colouration: Calypter margin and hairs grey. Posterolateral corner of frons brown, usually fading to yellow to base of outer or inner vertical bristle; orbital plate sometimes with thin brown margin tapering to anterior ori (few western specimens); back of head above foramen, ocellar tubercle and clypeus brown; venter of gena with thin brownish stripe that sometimes fades posteriorly; anterior margin of first flagellomere rarely appearing lightly infuscated. Scutum with complete lateral yellow stripe, sometimes with brown posterior mottling; katatergite sometimes with posterior margin to posterior half brown; anatergite dark below scutellum and paler lateral to scutellum, often with posterodorsal corner yellow. Pleuron yellow with ventral 2/3 of katepisternum, meron, variable markings on anepimeron and anteroventral corner of anepisternum brown; anepisternum sometimes predominantly brown along ventral margin; specimens from western North America sometimes darker with only dorsal 1/4 of anepisternum (as well as deep posterodorsal emargination), meron and katepisternum yellow; if only dorsal margin of anepisternum thinly yellow (rare), then lateral margin of frons infuscated, femora more extensively mottled dorsally or only yellow apically and distoventrally, lateral margin of scutum sometimes brownish postsuturally, laterotergites darker and abdomen entirely brown. Legs yellow with tibiae, tarsi and base of coxae light brown; base or dorsal base of hind and (less commonly) mid femora sometimes brown (if so, base of fore femur occasionally also brown); fore femur, and much less commonly mid and hind femora sometimes with outer-dorsal striations. Abdomen brown with lateral and sometimes posterior margins yellow; epandrium dark with dorsum perianal region usually yellowish.
Genitalia: Figs 5–9 View FIGURES 5–9 . Surstylus with single subapical spine. Phallus very short and weakly-sclerotized, with basiphallus faint and cylindrical, paraphallus pale, straight and rod-like, hypophallus well developed, and mesophallus distinct and appearing fused to distiphallus in ventral view; distiphallus with apical, basal and ventral surfaces more well-sclerotized, forming weak C-shape in profile. Ejaculatory apodeme pale and with base of blade and stem relatively dark and narrow, sometimes broader apically with corners more pointed.
Variation: One male and female from San Diego reared ex. Jacaranda entirely devoid of pigment. Rarely with lateral yellow stripes on scutum thinly continuing along posterior margin.
Hosts. L. sativae is a highly polyphagous species, although crop plants in the Cucurbitaceae, Leguminosae and Solanaceae appear to be favoured ( Spencer, 1973a)—see Table 1.
Californian specimens have been reared from the following: beans (including red kidney bean, “k.w. bean” and “beans”); clover; cucumber; squash, including banana squash; pumpkin; “melon”; Crenshaw melon; watermelon; tomato; petunia; “ex. potato tops with tuber moths”; wild mustard; Ambrosia acanthicarps ; Ambrosia psilostacha ; “ Cineraria Senecio x hybridus ”; Coreopsis ; Cucumis mello ; Datisca glomerata (upper surfacer serpentince mine); Dicoria canescens ; Jacaranda ; Medicago sativa ; Melilotus . Adults have also been collected on the following: beans (including castor bean, “black eyes”, “lima beans”, “ pink beans”, “pole beans” and “beans”); cantaloupe; Crenshaw melon; celery; cockle burr [= Xanthium strumarium ]; corn; cucumber; gound cherry; lettuce; wild mustard; mustard greens; radish; romaine lettuce squash; watermelon; zucchini; Baccharis ; Chenopodium ; Isomeris ; Iva axillaris ; Silybum marianum ; “ex. Verbena ”. Material, likely adults, have also been collected on “ mistletoe on Pinus sabiniana ”—if reared from mistletoe, this would be the first record of this species on the order Santalales *.
Range. USA: Alabama, California [widespread], Florida, Hawaii, Kansas, Louisiana, Maryland, New Mexico, Ohio [greenhouse], South Carolina, Texas. Neotropics. Introductions: Arabian Peninsula, Cameroon, China, Greece, Guam, India, Japan, Nigeria, Oman, Russia, Tahiti, Thailand, Turkey, Turkmenistan, Zimbabwe ( Dempewolf 2004, Deeming 2006, Tschirnhaus, pers. comm.) .
Type material. Liriomyza sativae : Holotype, ARGENTINA . "las larvas producen galerias en las hojas de la alfalfa en General Pico, Pampa; halladas por mi excelente colabodaro Juan Williason, xi.1937 ”, ex. Medicago sativa ( 1♀, Museu de la Plata, Buenos Aries, Argentina ) [Not examined]. Liriomyza canomarginis : Holotype, USA. Hawaii: Oahu, Kaimuki, 12.iv.1921, O.H. Swezey, ex. Indigofera sp. ( 1♀, BPBM) [Not examined]. Liriomyza guytona : Holotype, USA. Alabama: Auburn, 20.iv.1957, ex. beans, C.C. Freeman ( 1♂, USNM); Paratypes examined, USA. Alabama: Lee Co., Auburn, 25.iv.1957, Phaseolus sp. ( 2♂, EMEC). Liriomyza minutiseta : Holotype, USA. Hawaii: Oahu, Honolulu, 7.ix.1951, W.C. Mitchell, ex. tomato ( 1♀, BPBM) [Not examined]. Liriomyza munda : Holotype, USA. California: San Joaquin Co., Tracy, 22.ix.1949, L.L. Lewallen, ex. leaf of tomato ( 1♂, USNM); Paratypes examined, USA. California: San Joaquin Co., Tracy, 28.ix.1948, ex. larva Lycopersicon esculentum, Lot No. 175-1, L.L. Lewallen ( 2♂ 2♀, EMEC). Liriomyza subpusilla : Holotype, USA. Kansas: Manhattan, 14.x.1933, C.W. Sabrosky ( 1♂, USNM). Liriomyza pullata : Holotype, USA. Hawaii: Kanoa, Molokai, 3.iii.1929, O.H. Swezey, ex. Datura sp. ( 1♀, BPBM) [Not examined]. Liriomyza verbenicola : Holotype, USA. New Mexico: Las Cruces, ex. Verbena sp. ( 1♀, ZMHU) [Not examined].
Additional material examined. USA. California: 758♂ 1021 ♀ 51? [ CASC, CSCA, EMEC, SBMN, UCD, UCR, USNM] .
Comments. Liriomyza sativae is a highly polyphagous species and one of the most commonly encountered Liriomyza in agricultural regions in the United States. It is a highly invasive pest and has spread to most regions of the world excluding Australia. The external colouration of this species is incredibly variable and overlaps significantly with other regularly encountered species such as L. brassicae , L. eupatorii , L. helianthi , L. sabaziae and even the darker L. langei , almost always necessitating male dissections to confirm identifications. Scheffer and Lewis (2005; see also Scheffer (2005)) documented three highly divergent mitochondrial clades within L. sativae suggestive of the presence of cryptic species or host races. More recently, samples from South America have shown an additional fourth clade (Scheffer, pers. comm.). To date, globally invasive populations of L. sativae have all represented only one of the four mitochondrial clades, suggestive of real interclade biological differences (Scheffer, unpub. data).
Spencer (1981) incorrectly notes that the types of Liriomyza pullata , L. minutiseta and L. canomarginis , designated by Frick, are housed at the CASC. Originally deposited in the collection of the Hawaiian Sugar Planters’ Association, they would have been transferred to the BPBM along with the remainder of that collection (N. Evenhuis, pers. comm.).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Liriomyza sativae Blanchard
| Lonsdale, Owen 2011 |
Liriomyza guytona
| Freeman, C. C. 1958: 344 |
Liriomyza munda
| Frick, K. E. 1957: 61 |
Liriomyza pictella (Thompson)
| Frick, K. E. 1957: 66 |
Liriomyza propepusilla
| Frick, K. E. 1957: 62 |
| Frost, S. W. 1954: 73 |
Liriomyza pullata
| Frick, K. E. 1952: 509 |
Liriomyza canomarginis
| Frick, K. E. 1952: 511 |
Liriomyza minutiseta
| Frick, K. E. 1952: 512 |
Liriomyza verbenicola
| Hering, M. 1951: 43 |
Liriomyza subpusilla
| Frost, S. W. 1943: 255 |
Liriomyza sativae
| Palacios, T. R. E. & Romero N. & Etienne., J. & Koch., S. D. & Teran V., A. P. 2008: 14 |
| Scheffer, S. J. & Lewis, M. L. 2005: 181 |
| Rauf, A. & Shepard, B. M. & Johnson, M. W. 2000: 257 |
| Spencer, K. A. & Steyskal, G. C. 1986: 292 |
| Spencer, K. A. 1984: 23 |
| Spencer, K. A. 1983: 59 |
| Spencer, K. A. 1982: 27 |
| Spencer, K. A. 1973: 219 |
| Frick, K. E. 1959: 405 |
| Blanchard, E. E. 1938: 354 |