Plakortis symbiotica, Vicente, Jan, Zea, Sven & Hill, Russell T., 2016
|
publication ID |
https://doi.org/10.11646/zootaxa.4178.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:7A957617-C37C-41C8-9A8C-D7BB9178638C |
|
DOI |
https://doi.org/10.5281/zenodo.5617742 |
|
persistent identifier |
https://treatment.plazi.org/id/E622879F-FFDD-CC4F-15D9-FA08FE380C55 |
|
treatment provided by |
Plazi |
|
scientific name |
Plakortis symbiotica |
| status |
sp. nov. |
Plakortis symbiotica View in CoL sp. nov.
( Fig. 3 View FIGURE 3 ; Table 2 View TABLE 2 )
Plakortis sp. 2; Vicente et al. 2014 (ecology and symbiosis).
Type material. Holotype and type locality: USNM 1254650 About USNM , basibiont of Haliclona plakophila sp. nov., Old Buoy, La Parguera, Puerto Rico ( 17.9552° N, - 67.0532° W), 32 m depth, coll. Jaaziel García Hernández, October 15, 2015 GoogleMaps . Paratype USNM 1254649 About USNM , basibiont of X. deweerdtae, Old Buoy, La Parguera , Puerto Rico, ( 17.9552° N, - 67.0532° W), 32 m depth, coll. Jan Vicente, August 13, 2012 GoogleMaps .
Specimens examined for comparison (other than those described here). Plakortis halichondrioides : PHBH, Little San Salvador, Bahamas , PHPR, La Parguera, Puerto Rico .
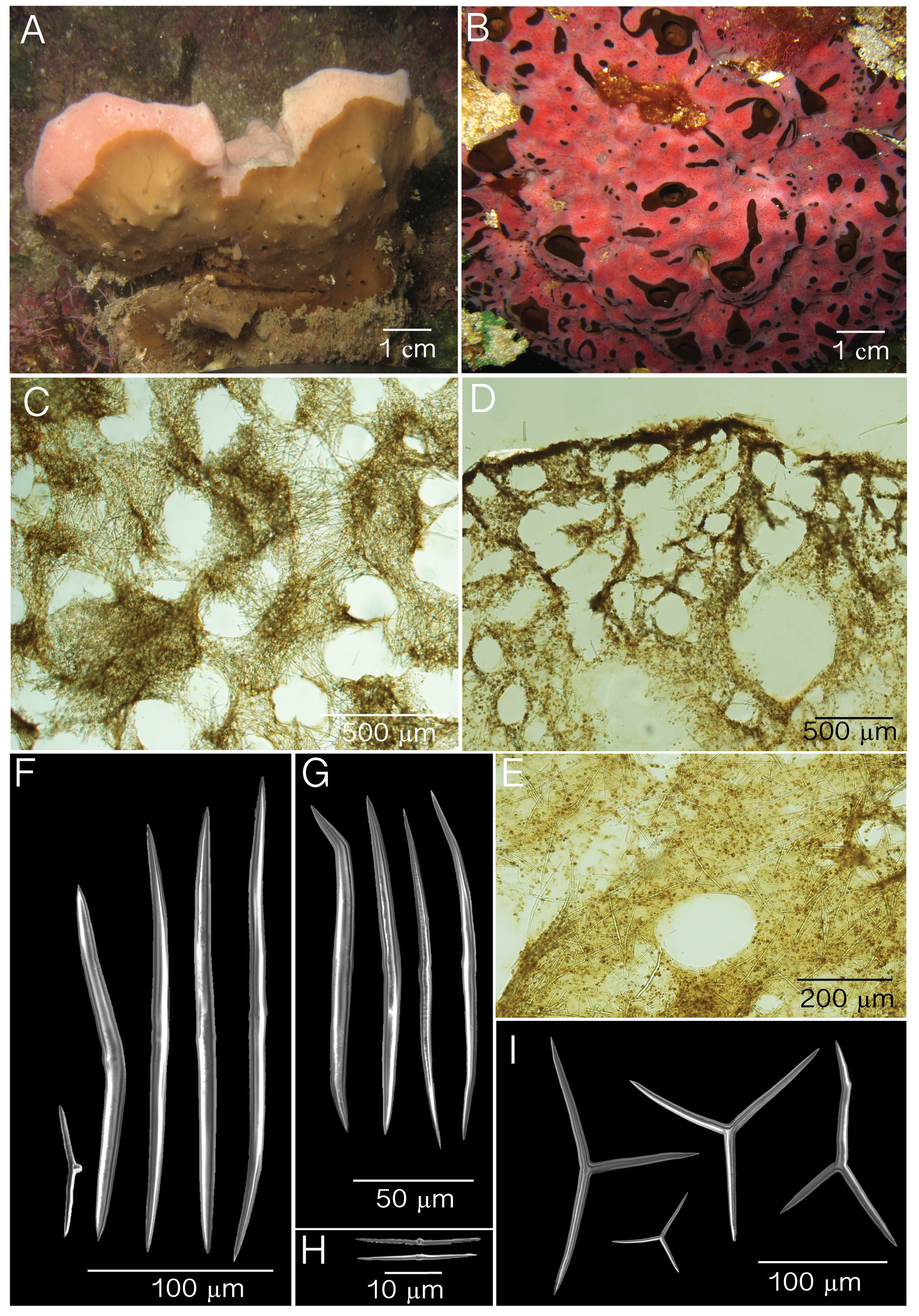
Diagnosis. Thickly encrusting cushions with a soft irregular surface and compressible body. Found as basibiont of X. deweerdtae but also of Haliclona plakophila sp. nov. In Puerto Rico, both X. deweerdtae and H. plakophila can be found on the same individual of P. symbiotica sp. nov. Both sponge epibionts grow as patches on the P. symbiotica body, never fully covering the basibiont as observed in P. deweerdtaephila , but can penetrate the basibiont body forming inner channels. Oscules are slightly elevated from the surface. Color can be dark green and dark brown in vivo and exudes a brown pigment when preserved in ethanol. Reticulated tangential ectosomal skeleton and reticulated choanosomal skeleton with lacunae present. Spicules consist of triods and diods of one size class.
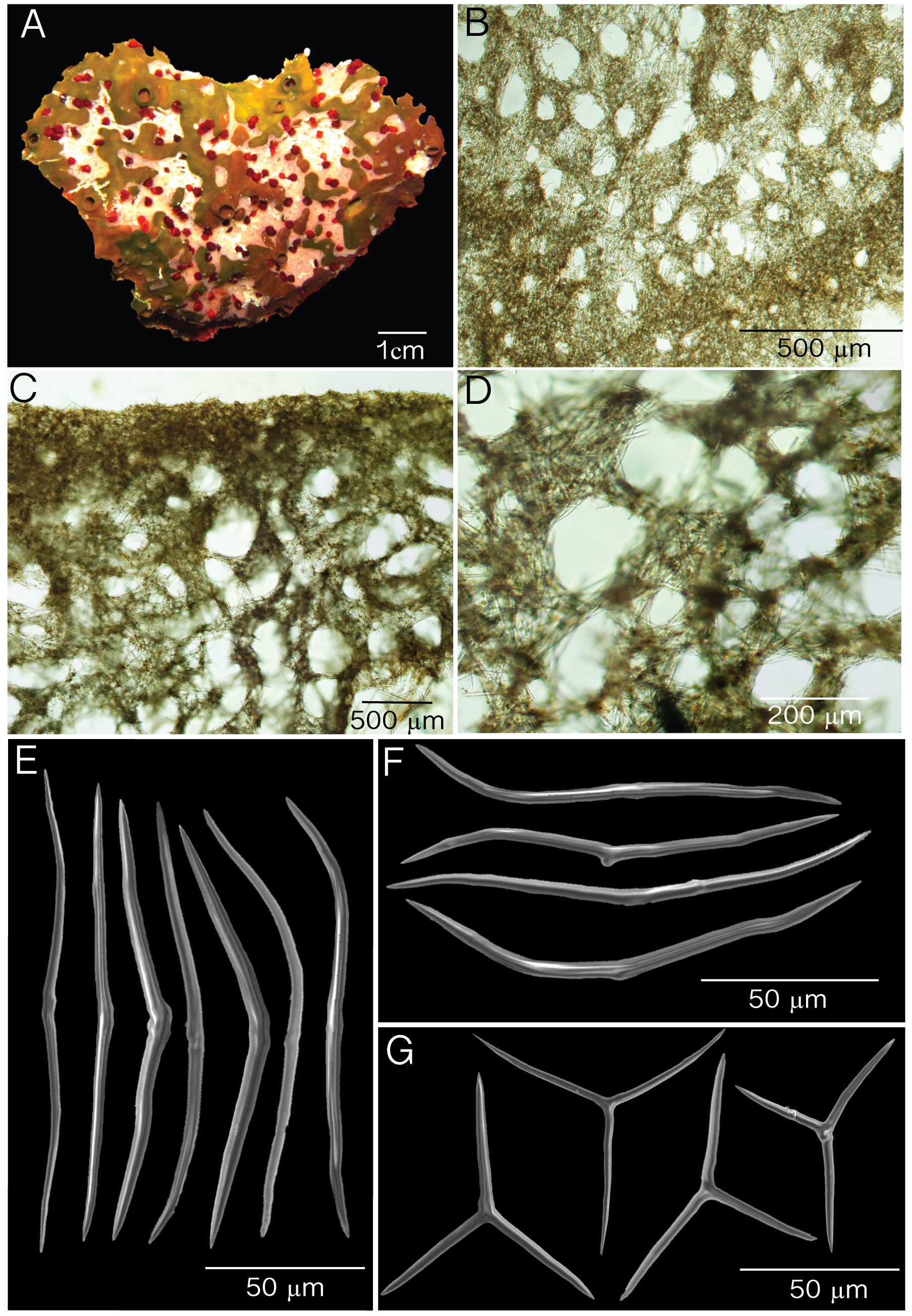
Description. Thick cushions 3× 30 cm by 1–3 cm in thickness ( Fig. 3 View FIGURE 3 A). Oscules are slightly elevated measuring 0.3–1.7 cm in diameter. Oscules in preserved specimens are contracted. External and internal color is dark brown; a dark brown pigment is exuded when preserved. Surface is smooth, soft and irregular where X. deweerdtae or H. plakophila sp. nov. grows. Consistency is compressible, and easily torn.
Skeleton. Ectosome is a disorganized tangential reticulation of diods and triods. Multispicular tracts are not well defined but form circular meshes, 43– 73 –121 µm diameter (N=20; Fig. 3 View FIGURE 3 B). Spicules barely break the surface of the ectosome. When X. deweerdtae or H. plakophila sp. nov. form inner channels within the choanosome of P. symbiotica sp. nov., the ectosome forms a barrier between the two sponge species as observed by Vicente et al. (2014, their Fig. 7 View FIGURE 7 E and F). The ectosome (60–70 µm thick) is dense and sometimes hard to distinguish from the choanosome. Subectosomal lacunae are present ( Fig. 3 View FIGURE 3 C). The choanosomal skeleton is dense but has an abundance of irregular circular meshes of varying diameters formed by a confused reticulation of diods and triods ( Fig. 3 View FIGURE 3 D).
Spicules. One size class of diods and triods. Diods are significantly sinuously bent, with a thick center. Ends are sharp and significantly bent ( Fig. 2 View FIGURE 2 E–F). Size (length x width): 72– 113.1 (±16.7)–142 µm x 2.2– 3.6 (±0.8)–5.0 µm ( Table 1). Triods are rare, Y-shaped, smooth, with sharp endings that are sometimes bent ( Fig. 2 View FIGURE 2 I): 20– 40.4 (±12.8)–71 µm long by 2.0– 3.3 (±0.7)–4.7 µm in width ( Table 1). Microrhabds, quasiamphiasters and spheres are absent.
Habitat and ecology. Sponge pairs are found on vertical walls (> 30 m), shaded side of pinnacles, and in reef cave habitats. Like Plakortis deweerdtaephila sp. nov., the new species has only been found either associated with X. deweerdtae or H. plakophila , never free-living ( Vicente et al. 2014). Sponge pairs have been documented from small recruits ( Fig. 3 View FIGURE 3 D in Vicente et al. 2014) and growth morphologies of sponge pairs remain stable for long periods of time (Supplementary Fig. 4 View FIGURE 4 in Vicente et al. 2014).
Distribution. Bahamas (Little Inagua) and Puerto Rico ( Mona Island, La Parguera, Desecheo) ( Figure 1 View FIGURE 1 B, D).
Taxonomic remarks. As in Plakortis deweerdtaephila sp. nov., the lack of microrhabds or quasiamphiasters in P. symbiotica sp. nov., places it within the P. simplex species group (see above, cf. Muricy 2011). P. symbiotica can be distinguished from all species of this complex by its association status with either haplosclerid, as well as by color, shape, size consistency and spicule composition. For example, P. symbiotica has an irregular surface with large oscules which sets it apart from other Plakortis spp. in this group. Unlike P. edwarsii , only large diods are present in P. symbiotica that form organized ectosomal meshes without cluttering opened circular spaces. Diods are also densely packed in both the ectosome and choanosome. P. symbiotica occasionally forms lacunae which are not present in P. da r i a e or P. insularis .
Other than P. deweerdtaephila , the only other species to associate with X. deweerdtae is P. symbiotica (but see remarks above regarding P. angulospiculatus as basibiont of X. deweerdtae from Belize, cf. Rützler et al. 2014). In Puerto Rico, H. plakophila is also an epibiont of P. s y m b i o t i c a. There are several morphological differences between P. deweerdtaephila and P. symbiotica . For example, P. s y m b i o t i c a has only large diods with small diods absent. The large diods are significantly more bent than in P. deweerdtaephila ( Fig. 2 View FIGURE 2 E, F). The ectosome has circular meshes with smaller diameters (43– 73 –121 µm; Fig. 3 View FIGURE 3 B) than the ectosomal circular meshes of P. deweerdtaephila ( 114– 205 –329 µm; Fig. 2 View FIGURE 2 C). The ectosome is denser and harder to differentiate in cross-sections of P. symbiotica than of P. deweerdtaephila . Subectosomal lacunae are present but much fewer than in P. symbiotica ; the choanosome also appears to have more circular meshes than P. symbiotica .
As for P. deweerdtaephila , P. symbiotica spicules were compared with those of P. halichondrioides by Vicente et al. (2014), and shown to be larger in the latter. Our direct comparisons with a specimen of P. halichondrioides from Puerto Rico show that P. s y m b i o t i c a can be distinguished by having smaller spicules and like P. deweerdtaephila spicules never cross the surface of the ectosome or the open spaces of the circular meshes. Circular meshes are therefore better defined in P. deweerdtaephila than in P. halichondrioides .
Phylogenetic analysis. Our phylogenetic analysis used maximum likelihood to compare partial sequences of the cytochrome b ( cob) and c ( cox1) genes of our new Plakortis spp. to other homoscleromorph sponge sequences deposited in GenBank. Our analysis confirmed that P. deweerdtaephila sp. nov. and P. symbiotica sp. nov. are more closely related to one another than to any other homoscleromorph or Plakortis species ( Fig. 4 View FIGURE 4 ). Sequence homology between both species was 93% for cob and 94% for cox1 and formed a clade that was supported with bootstrap values (85 and 78% respectively). On the other hand, these differences are enough to support their status as separate species, which is further supported by the morphological differences outlined above. The closest relatives of the new species were P. simplex and P. dariae with a 92% sequence homology for both genes. Despite having similar morphological features compared to P. e dw ard s i, this species turned out to be more closely related to P. halichondrioides and P. angulospiculatus in the phylogenetic analysis of both genes (Ereskovsky et al. 2013). Support values in the phylogenetic analysis for the P. symbiotica and P. deweerdtaephila clades suggests that the symbiotic association of these sponges with other sponges is perhaps an ancestral, and even a synapomorphic character.
Etymology. The name symbiotica denotes the tendency of the new species to associate with X. deweerdtae and H. plakophila sp. nov.
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |