Caenorhabditis brenneri, Sudhaus & Kiontke, 2007
|
publication ID |
https://doi.org/10.11646/zootaxa.1456.1.2 |
|
publication LSID |
lsid:zoobank.org:pub:1C9D8919-DDA2-496E-8277-D8FB7DCA92CB |
|
persistent identifier |
https://treatment.plazi.org/id/E67087B7-3F2C-FFDD-1F83-4EA1C02FFAA8 |
|
treatment provided by |
Felipe |
|
scientific name |
Caenorhabditis brenneri |
| status |
sp. nov. |
Caenorhabditis brenneri sp. n. 1 ( Figs 2–4 View FIGURE 2 View FIGURE 3 View FIGURE 4 )
= Caenorhabditis remanei auct. 2 = C. n. sp. 4 in Sudhaus & Kiontke (1996) and Kiontke & Sudhaus (2006) =? Rhabditis teres apud Schuurmans Stekhoven, 1951, nec (Schneider, 1866)
Measurements see Table 2.
With the characters of the stem species pattern of the Elegans group. The following details were observed in strain SB129 and might be of diagnostic value.
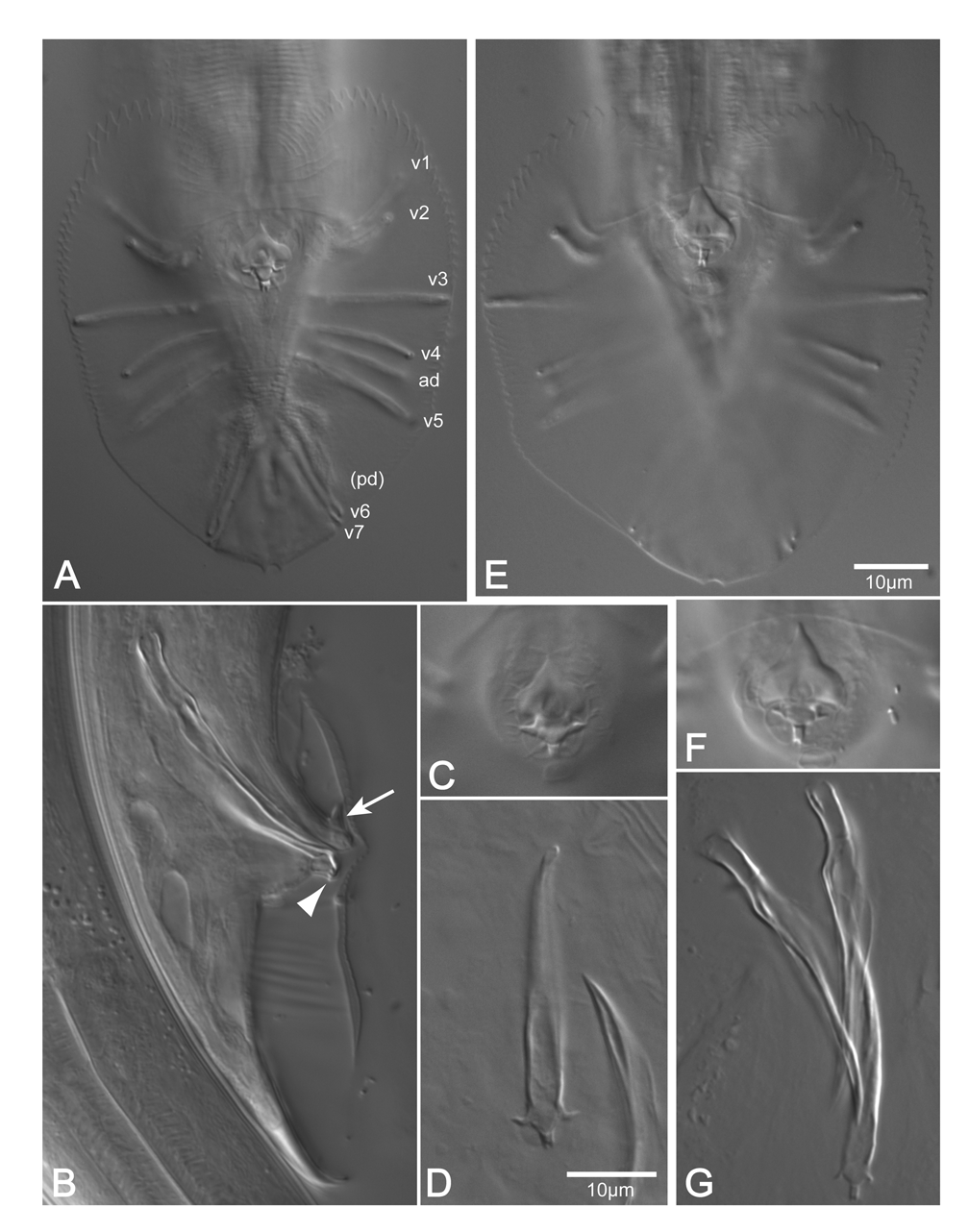
Adult: Stoma length about 1.5–2 times the diameter in the lip region. Stoma diameter sexually dimorphic; the ratio of stoma length to width is about 5 in the Ƥ and about 7 in the ♂. Corpus intima finely transversely striated. Nerve ring at anterior third of isthmus. Position of deirids variable, slightly anterior or posterior to excretory pore. Position of postdeirids at approximately 66 % of body length in the Ƥ, and 81 % in the ♂. Width of lateral field about 3 µm or 1/7 to 1/12 of body width; the longitudinal cuticular ridges extend from level of median pharynx bulb to posterior the anus in the Ƥ, and nearly to the distal end of the spicules in the ♂. Chromosome number 1n=6.
Female: Lateral canals of secretory-excretory system extend to one ABW posterior of the anus. The position of the vulva appears exactly in the middle between pharynx end and anus; the distance between vulva and anus is 92–124 (104) % the distance from pharynx end to vulva. The posterior gonad arm comprises 88–118 (101) % of the anterior one. At maximum 7 embryos in each uterus, sometimes developed to J1 ready to hatch.
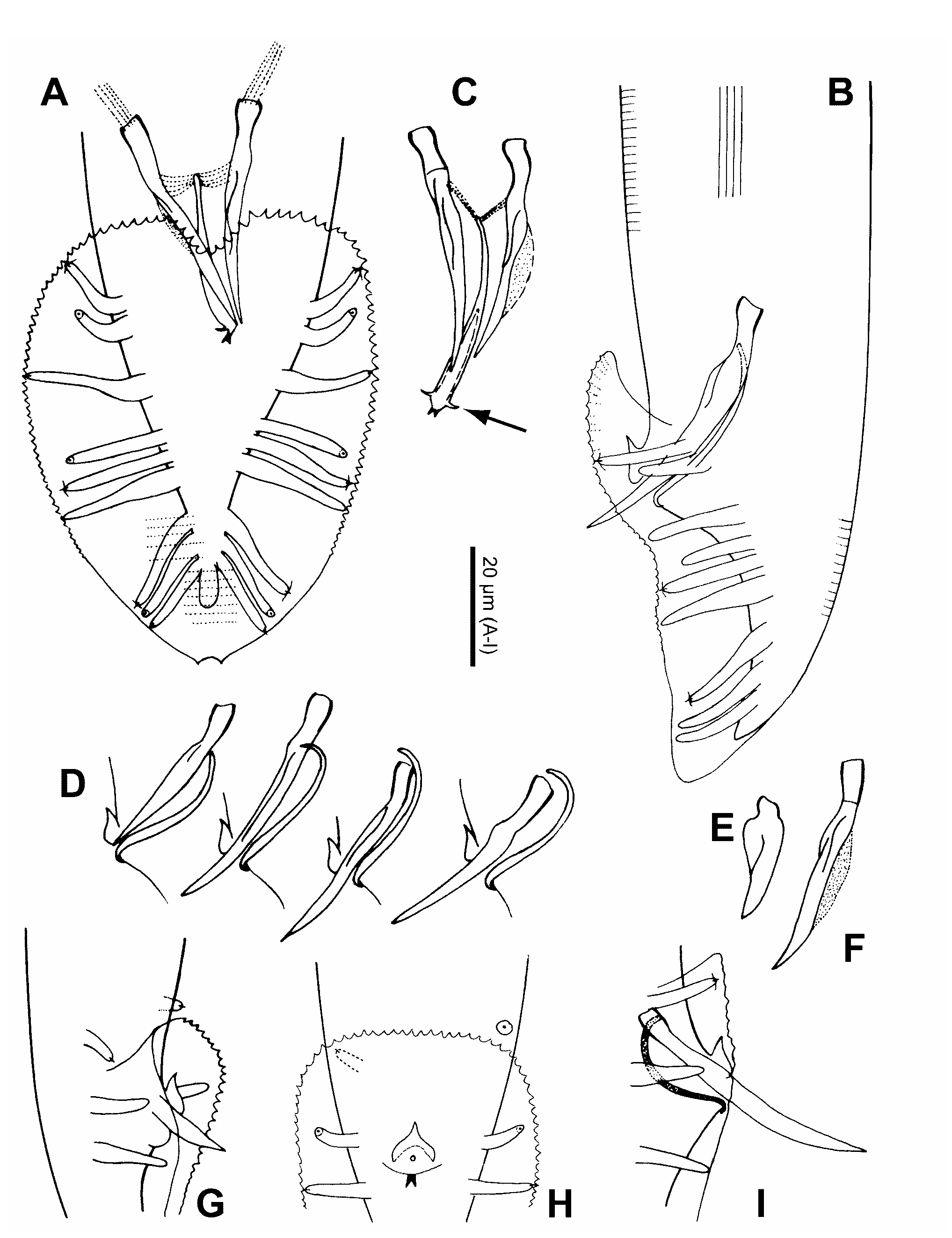
Male: The distance between the pharynx end and flexure of the testis is 56–159 (101) µm or 71–154 (116) % of testis flexure length. Bursa as in the stem species. In degenerating specimens GP7 becomes slightly bottle shaped like GP2 , GP3 and GP6 . GP4 a little shorter than GP5 . Spicules can differ in length by about 3 %. The dorsal velum on the spicule terminates one fifth of a spicule length from distal tip. Proximal end of gubernaculum appears slightly swollen where muscles insert, the distal projections (ears) are curved like claws or hooks, weakly sclerotized, whereas the forked terminal piece is heavily sclerotized, visible as two conspicuous points in ventral view of the intact ♂ .
Aberrations: One ♂ with testis left of intestine. In one case both spicules and the gubernaculum were stunted (a phenotype which is observed in C. elegans when the muscles required for morphogenesis of the
1. This species is dedicated to Sydney Brenner, who, 40 years ago, initiated the fundamental work on C. elegans .
2. In papers published before 1995/96 ( Binder et al. 1992, Cangiano & La Volpe 1993, Felsenstein & Emmons 1988, Fitch et al. 1995, Fodor & Timar 1989, Fodor et al. 1989, Hekimi 1990, Hodgkin 1984, Jones & Schedl 1995, Kloek et al. 1996, Lee et al. 1993, Sedensky et al. 1994, Thomas & Wilson 1991, Youngman et al. 1996) this nematode was designated as " C. remanei " referring to strain CB5161 isolated in Trinidad and deposited at the CGC in 1988. Of the sequences deposited at GenBank under the name C. remanei , six belong to C. brenneri sp. n.. These sequences have the accession numbers U75913 View Materials , U01838 View Materials and U48292 View Materials –5. Some C. remanei sequences are deposited under the synonym C. vulgaris . proctodeum are damaged or absent; Sulston et al. 1980); in another case, the right spicule was abnormally short and broad, measuring 21 µm whereas the left spicule was 36 µm long ( Fig. 3E, F View FIGURE 3 ). In one ♂ GP4 missing on the left hand side. In another ♂ the arrangement of GPs as usual on the left side (GP1 and GP2 clustered), but on the right side GP1 was separated from GP2 and shifted to the anterior margin of the closed velum ( Fig. 3I View FIGURE 3 ). Once, both GP1 were positioned anterior of the closed bursa velum, standing slightly asymmetrically ( Fig. 3G, H View FIGURE 3 ). This aberration is of some evolutionary significance. It demonstrates that a bursal papilla which is integrated in a proximally closed velum can disassociate from the velum and secondarily adopt a prebursal position. Such a scenario was discussed for the evolution of the prebursal genital papillae in Pelodera kolbi (Sachs, 1950) within the Coarctata -group ( Sudhaus 1976, p. 25; Sudhaus & Fitch 2001, p. 23). Type locality and habitat Bohorok in Sumatra (SB129). Compost-like material, mostly banana leaves. Type material
Holotype (male, no. 11232) and paratypes (no. 11233) deposited in the collection of the Museum für Naturkunde der Humboldt-Universität zu Berlin, Germany; further paratypes in Naturhistoriska Riksmuseet Stockholm, Sweden; Laboratorium voor Nematologie, Landbouwhogeschool, Wageningen, the Netherlands; USDA, Nematology Laboratory, Beltsville, Maryland, USA; collection of Prof. W. Sudhaus, Institut für Biologie , FU Berlin, Germany.
Comparison and diagnosis
From a comparison with C. brenneri sp. n., we can exclude C. briggsae and C. elegans , which are self-fertilizing protandrous hermaphroditic species with a very low percentage of residual males. C. brenneri sp. n. differs from C. clavopapillata , which exhibits a group of only three (instead of 1+3 = 4) genital papillae (GP) immediately posterior of the cloaca. Since the first and third GP of this group are basally swollen, they can be identified as GP3 and GP6. Therefore, a likely interpretation is that GP4 disappeared by fusion with GP3, as it is often observed in C. briggsae . Despite thorough analyses, no clear difference could be established in morphometric data between C. brenneri sp. n. and C. remanei which are isolated post mating. Moreover, there are almost no differences in morphology. During an extensive survey of morphological characters we found slight differences in a number of characters between and within isolates of the two species. However, these differences do not constitute clear differences between the two species. The characters include the location of deirids in relation to the cervical pore, the number of teeth along the bursa margin between GP1 and GP3 and between GP3 and GP6, and the conspicuousness of the forked process at the distal end of the gubernaculum. An exception is the shape of the lateral processes at the distal part of the gubernaculum which we call ears. These projections are shaped like small thorns in C. remanei ( Fig. 4F View FIGURE 4 ) but like elongate curved claws in C. brenneri sp. n. ( Fig. 3C View FIGURE 3 and 4C View FIGURE 4 ). The sibling species are further distinguished by the length of GP4 relative to GP5: GP4 is slightly shorter than GP 5 in C. brenneri sp. n., whereas both GPs are of almost equal length in C. remanei . GP4 is longer than GP 5 in C. briggsae and C. elegans .
C. brenneri sp. n. must be compared with two further species, C. formosana from Taiwan ( Changhua Country) and C. oncomelaniae from Japan (Kyushu). Both were described as associates of the snail Oncomelania hupensis Gredler. Based View in CoL on the descriptions, we would not hesitate to synonymize these two species, were they not studied by the same authors at the same time ( Yokoo & Okabe 1968). Unfortunately, the authors did not conduct cross mating experiments. Neither species was found again even though a large body of field work was done on the snail Oncomelania hupensis View in CoL , it being the intermediate host of Schistosoma View in CoL . Unfortunately, Oncomelania View in CoL snails can not be examined anymore at or near the type locality of C. oncomelaniae in the prefecture Saga of Kyushu because some years ago the snails were eradicated in this area (Toyoshi Yoshiga, pers. comm.). We know of only three isolates of Caenorhabditis from Oncomelania View in CoL in Taiwan (PS1185 and PS 1186 W. Kelley Thomas, pers. comm.) and Japan ( Hokkaido, sampled 1995 by Mitsuhiko Asakawa for W. S.), and all were identified as C. briggsae . These records are thus incompatible with the gonochoristic C. formosana and C. oncomelaniae . Since there is thus no new material of these gonochoristic Caenorhabditis species from East Asia, we rely on the original descriptions by Yokoo & Okabe (1968) to argue that C. brenneri sp. n. is different from C. formosana and C. oncomelaniae . Most characters and measurements are overlapping, except for the length of the spicules (< 38 µm in C. brenneri sp. n. as opposed to> 40 µm in C. oncomelaniae and> 45 µm in C. formosana ). C. formosana differs from C. brenneri sp. n. in possibly basally fused GP4 and GP5, and in the more anterior position of the phasmids in the female (1.5 times ABW posterior anus vs.>2 times ABW in C. brenneri sp. n.), assuming that this is not an artifact in the photograph (Fig. 6.4) or an error in the drawing ( Fig. 5.4 View FIGURE 5 ) in Yokoo & Okabe (1968). C. oncomelaniae is larger than C. brenneri sp. n. ( ♂ body length 1020–1430 µm vs. < 950 µm; b = 5.7–7.8 vs. <5.6; gubernaculum length 30–37.5 µm vs. < 30 µm), the anterior gonad of the C. oncomelaniae female is always shorter than the posterior gonad, whereas both are of almost equal length in C. brenneri sp. n., the anterior ovary comprises 14–21 % of body length in C. oncomelaniae vs. 18–31 % in C. brenneri sp. n.; finally, the nerve ring of C. oncomelaniae is located in the posterior part instead of the anterior part of the isthmus. As a potential additional difference, C. formosana and C. oncomelaniae were both isolated from temperate locations north of and at the tropic of cancer, whereas C. brenneri sp. n. is only known from tropical locations. Even though Okabe & Shiraishi (1971) were highly successful in infesting snails with C. oncomelaniae in the laboratory, there are two reasons not to consider the association of C. formosana and C. oncomelaniae with Oncomelania View in CoL as an ecological difference to C. brenneri sp. n.. First, the records of C. formosana and C. oncomelaniae are unique and could not be repeated as yet; second, preliminary results in our laboratory show that it is easy to infest snails and slugs with different rhabditids, including Caenorhabditis . So far there is no evidence for a specific association of Caenorhabditis species with snails in Eastern Asia.
Ecology and biology
C. brenneri sp. n. was only found in anthropogenic habitats (plantations, agricultural land, plant nurseries, gardens) in rotting plant material, rich soil and dung. The species can be cultured on organic material of various kinds, and on nutrient agar. Because of the waving behavior of the dauerlarvae—single and in plaits with up to 50 individuals—an unspecialized phoretic association is expected. In the laboratory, phoretic transport by the predatory mite Hypoaspis miles (Berlese) was observed. Exceptional within Caenorhabditis is the desiccation tolerance of this species. Dauerlarvae can be kept in dry substrate for 11.5 months at 8 º C, for 7.5 months at room temperature, and for 4.5 months at 32 º C. In tap water dauer larvae lived for 8 months at 8 º C and for 160 days at 16 º C.
Gonochoristic; on average 48.1 % of adult progeny of single females in drops of nutrients were males (n = 5288), spanning between 40.3 and 54.9 % within the progeny of one female (n = 25). Copulation can occur in liquid medium. Initially, the male coils around the female with its posterior end. Later, mating males and females adopt a parallel position, equally frequently of the λ- or Y-type (14: 15). At maximum, 7 embryos were observed in each uterus. Mostly oviparous, sometimes juveniles hatch within the uterus. The progeny of one female is between 103 and 309 (187 ± 62; n = 29). Once 415 offspring were counted (females with fewer than 100 progeny were disregarded). One C. brenneri sp. n. ♀ which was mated with C. remanei ♂♂ laid 234 degenerating eggs, and one C. remanei ♀ laid approximately 100 eggs after mating with C. brenneri sp. n. ♂♂ ( Table 1). Virgin females lived 3–40 (14.3 ± 8.1) days (n = 30), and reproducing females lived 3–43 (12.5 ± 7.4) days (n = 55). Males lived 3–25 (11 ± 4.7) days (n = 139).
| USDA |
United States Department of Agriculture |
| FU |
Fudan University, Department of Biology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Caenorhabditis brenneri
| Sudhaus, Walter & Kiontke, Karin 2007 |
C. brenneri
| Sudhaus & Kiontke 2007 |
C. brenneri
| Sudhaus & Kiontke 2007 |
C. brenneri
| Sudhaus & Kiontke 2007 |
C. brenneri
| Sudhaus & Kiontke 2007 |
C. brenneri
| Sudhaus & Kiontke 2007 |
C. brenneri
| Sudhaus & Kiontke 2007 |
C. brenneri
| Sudhaus & Kiontke 2007 |
C. brenneri
| Sudhaus & Kiontke 2007 |
C. brenneri
| Sudhaus & Kiontke 2007 |
C. brenneri
| Sudhaus & Kiontke 2007 |
Oncomelania
| Gredler 1881 |
Oncomelania
| Gredler 1881 |
Oncomelania
| Gredler 1881 |
Rhabditis teres
| Schneider 1866 |