Litoria kroombitensis, Hoskin, Conrad J., Hines, Harry B., Meyer, Ed, Clarke, John & Cunningham, Michael, 2013
|
publication ID |
https://doi.org/10.11646/zootaxa.3646.4.6 |
|
publication LSID |
lsid:zoobank.org:pub:C038F8A1-BD93-4FF1-B52D-02F650371733 |
|
DOI |
https://doi.org/10.5281/zenodo.6153686 |
|
persistent identifier |
https://treatment.plazi.org/id/E87B87D4-DE1E-1540-1DC6-2D9C6F3CFDCA |
|
treatment provided by |
Plazi |
|
scientific name |
Litoria kroombitensis |
| status |
sp. nov. |
Litoria kroombitensis sp. nov.
Kroombit Treefrog
Figs 2 View FIGURE 2 A–C, 3–6 & 10
Holotype: J91640 View Materials , adult male, collected at Kroombit Ck, Kroombit Tops (24°23ʹ0 3ʺS, 151°00ʹ0 5ʺE), south-east Queensland, by H. B. Hines & C. J. Hoskin, on 7 December 2010.
Paratypes: J40160 View Materials , J40162 View Materials , J40163 View Materials , J40164 View Materials , Kroombit Ck crossing, Ubobo Rd, Kroombit Tops (24°22ʹS, 151°01ʹE); J42167 View Materials , Kroombit Tops (24°22ʹS, 151°01ʹE); J42184 View Materials , Kroombit Tops (24°22ʹS, 151°01ʹE); J42474 View Materials , Beauty Spot 98, Kroombit Tops (24°22ʹS, 151°01ʹE); J42475 View Materials , Beauty Spot 98, Kroombit Tops (24°22ʹS, 151°01ʹE); J42747 View Materials , Three Moon Ck, Kroombit Tops (24°22ʹS, 151°01ʹE); J42748 View Materials , Three Moon Ck, Kroombit Tops (24°22ʹS, 151°01ʹE); J72662 View Materials , Kroombit SF (24°23ʹ0 9ʺS, 151°00ʹ11ʺE); J72664 View Materials , Kroombit SF (24°23ʹ0 9ʺS, 151°00ʹ11ʺE); J72665 View Materials , Kroombit SF (24°23ʹ0 9ʺS, 151°00ʹ11ʺE); J72666 View Materials , Kroombit SF (24°22ʹ0 7ʺS, 150°59ʹ50ʺE); J86681 View Materials , Munholme Creek headwaters, Kroombit Tops NP (24°24ʹ55ʺS, 151°02ʹ19ʺE); J91638 View Materials , Kroombit Ck crossing, Kroombit Tops (24°23ʹ0 3ʺS, 151°00ʹ0 5ʺE); J91639 View Materials , Kroombit Ck crossing, Kroombit Tops (24°23ʹ0 3ʺS, 151°00ʹ0 5ʺE); J91641 View Materials , Kroombit Ck crossing, Kroombit Tops (24°23ʹ0 3ʺS, 151°00ʹ0 5ʺE); J91658 View Materials , Kroombit Ck crossing, Kroombit Tops (24°23ʹ0 3ʺS, 151°00ʹ0 5ʺE).
Diagnosis. A small (< 45 mm SVL) green or greenish-brown frog with distinct, rounded finger and toe pads; a thin gold line running from naris over eye and tympanum to above forelimb; white gilding on the trailing edges of the fore- and hindlimbs; unpatterned orange posterior thighs; a gold iris; a blunt, gently rounded snout in profile; a smooth dorsum; and a mating call consisting of a short whine followed by one or more chirps. Litoria kroombitensis sp. nov. can be distinguished from similar congeners by a combination of the following traits: body size (male SVL 28.0– 32.1 mm; female SVL 31.7–43.5 mm); head shape (HW/HL 1.22–1.40); upper lip pattern typically consisting of prominent white markings broken by a darker bar below the front of the eye; thin gold line bordered below by a dark band or line extending from nare to above forelimb; prominent white gilding on the trailing edges of the fore- and hindlimbs; fairly even green or greenish-brown dorsum; mating call consisting of whine followed by one or more chirps, with the whine being short (0.160– 0.229 s) and of fast pulse rate (500–656 pulses/s), and the chirp being of slow pulse rate (34–48 pulses/s). Litoria kroombitensis sp. nov. is also genetically distinct from congeners, for example being a highly divergent monophyletic lineage for 16S mtDNA (see Genetics).
Etymology. Referring to the restriction of this species to Kroombit Tops, with the - ensis extension being latin for ‘belonging to’. The epithet is to be treated as a noun in apposition.
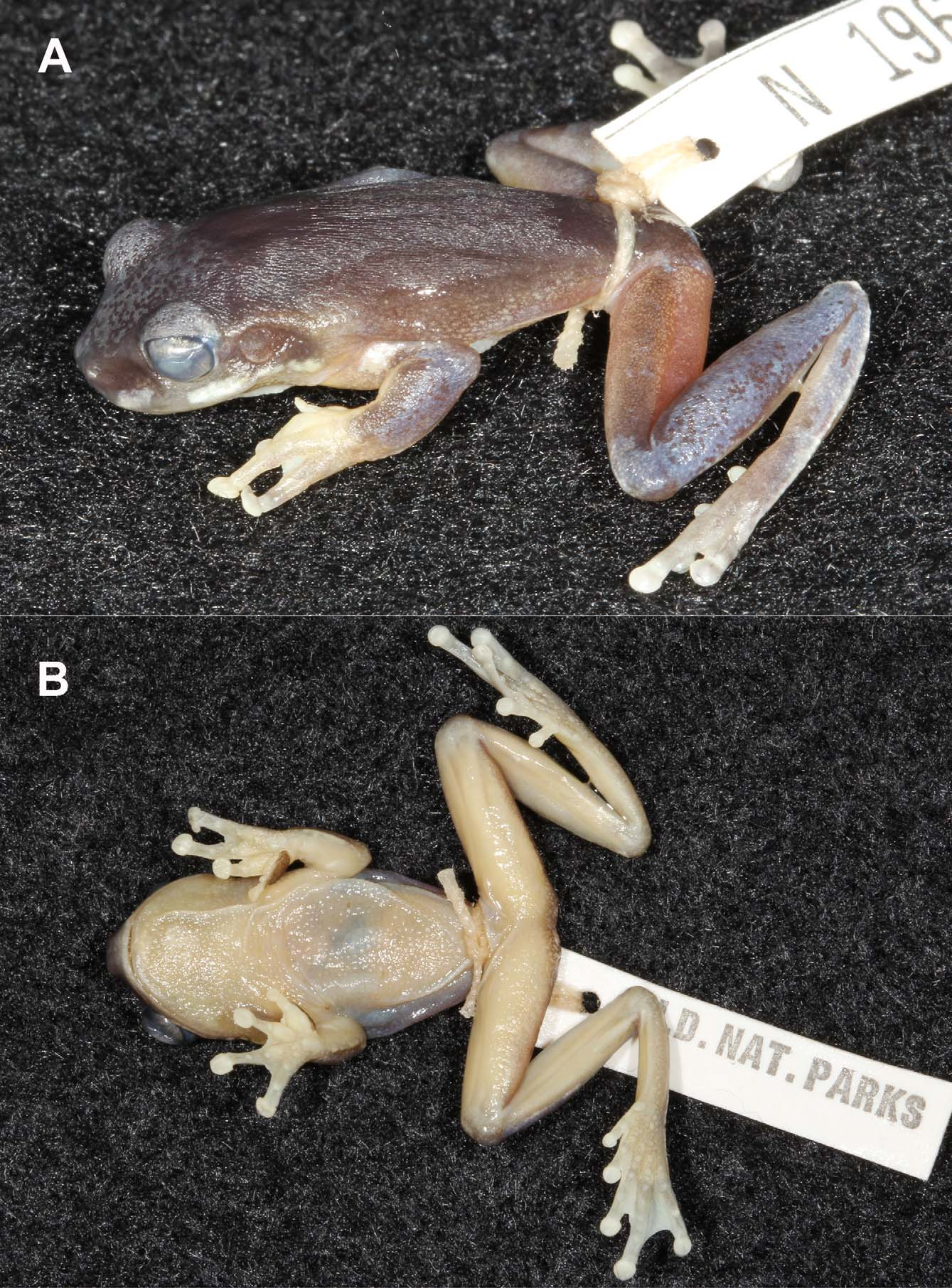
Description of holotype ( Fig. 3 View FIGURE 3 ). J91640 View Materials ; male. Measurements (mm): SVL 29.3; HLL 15.0; FL 7.2; HW 10.4; HL 8.3; EN 2.9; IN 2.1; ED 2.9; 3DW 1.4. Head: Broad and flattened, equal width to body; snout truncate in dorsal view and blunt and gently rounded in profile, canthus rostralis angular, loreal region steep, nostrils much closer to tip of snout than to eye, nostrils directed dorso-laterally; eye very large, diameter equal to eye to naris distance, horizontal pupil; internarial distance less than distance from eye to naris (EN/IN: average 1.42, range 1.28–1.59); tympanum moderate size and distinct. Body: Inversely triangular; urostyle indistinct; cloaca positioned immediately below urostyle, oriented posteriorly, no ornamentation. Limbs: Hindlimbs long and slender; forearms broad; pale distinct ridge (gilding) along the trailing edge of the forearm, on the heel and along the trailing edge of the foot; basal webbing on fingers, connecting the proximal phalanges (webbing formula: I 1–1 ½ II 1 ½–2 III 2–1 ½ IV); toes nearly fully-webbed (webbing formula: I 1–1 II 0– 1 III 0–1½ IV 1 –0 V); fingers long, relative length III>IV>II>I (II and IV about equal in length), finger discs fleshy and rounded, obviously expanded from penultimate phalanx, first finger short with disc obviously expanded; small, rounded subarticular tubercles under joints of fingers; a broad, long and ovoid, black nuptial pad (heavily stippled) covers the base and inside of the first finger; indistinct low, rounded tubercle present in the centre of the base of the palm; relative length of toes IV>V>III>II>1 (III and V about equal in length), rounded toe discs expanded from penultimate phalanx, disc on first toe expanded; small, oval inner metatarsal tubercle; low, rounded outer metatarsal tubercle; small, rounded subarticular tubercles under joints of toes; discs on fingers larger than discs on toes. Skin: Ventral surface of chest and belly coarsely granular; throat and undersides of thighs and forearms smooth, pectoral fold absent; dorsal surface of body, head and limbs smooth to finely granular; distinct postorbital skin fold extending along dorsolateral surface to above forelimb. Colour pattern in life: ( Fig. 2 View FIGURE 2 B). Dorsal surfaces of head, body and legs green, suffused with coppery brown. Several tiny black dots scattered on back and top of head. Broad dark grey band extends from tip of snout, through naris to eye and then extends above tympanum and becomes diffuse above forelimb. From behind eye this band is bordered above by a thin gold line. Prominent white along upper lip from below naris to below tympanum, broken by grey bar below front of eye. Tympanum green, smudged with brown. Iris copper-coloured. Copper colouration along the top-line of the folded hindlimb and to a lesser degree on the leading edge of the forearm. Thin white edge (gilding) to the trailing edge of the fore- and hindlimbs. Top of hands and feet pale with grey mottling; top of discs translucent pale yellowish grey. Nuptial pad black. Flanks merging from green-brown dorsal colour to pale ventral colour, and finely spotted with white. Chin, chest creamy white; belly a pale reddish colour. Posterior thigh orange-red. Groin orange with a purple hue. Axilla orange. Ventral surfaces of hind and forelimbs orange-red; undersides of hands and feet yellowish orange. Colour pattern in preservative ( Fig. 3 View FIGURE 3 ): Dorsum greyish brown overlain with greyish blue on forehead and top of eyes and on top of forelimbs, lower legs and feet. Orange tinge to posterior thigh. Top of feet and hands pale. Thin white line on trailing edge of forearm and heel and trailing edge of foot. Darker dorsum merges gradually on flanks to creamy undersides. Ventral colour cream. Pale edge to upper lip broken below front of eye. Pale upper lip markings extend as broken line below tympanum to forelimb. Tympanum dark. Groin as for flanks (grey-cream). Eye copper-grey with fine black venation. Urostyle pale.
Description of type series (N = 20, 15 males and 5 females). See Table 1 View TABLE 1 for measurements and proportions of type series. Head: Broad and flattened, equal width to body; snout truncate in dorsal view and blunt and gently rounded in profile, canthus rostralis moderately angular to angular, loreal region steep, nostrils much closer to tip of snout than to eye, nostrils directed dorso-laterally; eye very large, diameter greater than or equal to eye to naris distance, horizontal pupil; internarial distance less than distance from eye to naris; tympanum moderate size and distinct. Body: Slender and tapering in males, rotund in females; urostyle moderately prominent to indistinct; cloaca positioned immediately below urostyle, oriented posteriorly, often pale ornamentation. Limbs: Hindlimbs long and slender; forearms robust; pale distinct ridge (gilding) along the trailing edge of the forearm, on the heel and along the trailing edge of the foot; basal webbing on fingers, connecting the proximal phalanges; toes nearly fully-webbed; fingers long, relative length generally 3>2>4>1 (2 and 4 about equal in length), finger discs fleshy and rounded, obviously expanded from penultimate phalanx, first finger short with disc obviously expanded; in males, base of thumb swollen and covered with a heavily stippled area forming a prominent brown or black nuptial pad on the inner base of the first finger; prominent small, round subarticular tubercles under fingers; indistinct low, rounded palmar tubercle on some individuals, several small, low tubercles on others; relative length of toes generally 4>3>5>2>1 (3 and 5 about equal in length), rounded toe discs expanded from penultimate phalanx, disc on first toe expanded; prominent small, round subarticular tubercles under toes; small oval inner metatarsal tubercle; moderately distinct to indistinct ovoid or round outer metatarsal tubercle; discs on fingers larger than discs on toes. Skin: Ventral surfaces granular to finely granular, typically finer under chin and generally smooth under forelimbs and chest; dorsal surface of body, head and limbs smooth to finely granular; distinct supratympanic skin fold extending from behind eye to forelimb. Colour pattern in preservative: Dorsum of males greyish brown overlain with greyish blue on forehead and top of eyes and on top of forelimbs, lower legs and feet. Dorsum of females bluish grey. Posterior thigh washed orange or pinkish. Top of feet and hands pale. Distinct to indistinct thin white line on trailing edge of forearm and heel and trailing edge of foot. Darker dorsum merges gradually on flanks to creamy undersides. Ventral colour cream or white. Pale edge to upper lip, broken below front of eye by dark band from eye to lip. Pale upper lip markings extend as broken line below tympanum to forelimb. Tympanum dark. Groin as for flanks (grey-cream). Iris dark or copper-grey with fine black venation. Urostyle often pale.
Colour pattern in life ( Fig. 2 View FIGURE 2 A–C). Dorsal surfaces of head, body and legs typically uniform bright green, dark green or olive brown, sometimes brown or two-tone green and brown (e.g. J91640 View Materials ). Sometimes tiny black dots scattered on back (e.g. J91640 View Materials ). Thin gold line extends from nare, over eye and tympanum, to above forelimb. Bordered below by a dark band, typically a broad dark grey or brown band, but sometimes a narrow black line (e.g. J91613 View Materials ). Black band below the gold is completely absent (or reduced to flecks) on some individuals (e.g. J91611 View Materials ). Broken band of white markings along upper lip, extending from below nare to behind jaw angle. White markings typically prominent, especially from below centre of eye to below tympanum. In many individuals white markings extend to above insertion of forearm and onto upper surfaces of forearm (e.g. J91613 View Materials ). In some females, white lip markings are reduced to thin broken line (e.g. J91611 View Materials ). Typically a prominent dark bar (grey, brown or green) extends from below front of eye to lip, breaking up the white upper lip band. Lip bar generally more prominent in males. White markings and bar on lip are very prominent on subadults ( Fig. 4). Tympanum usually green, rarely brown; typically matching the laterally adjacent skin colour, making the tympanum indistinct. Iris gold or copper-coloured. Typically a thin gold line along the topline of the folded hindlimb and often also on the leading edge of the forearm. Thin but prominent white edge (gilding) to the trailing edge of the fore- and hindlimbs. Top of hands and feet pale with grey mottling; top of discs translucent yellow or pale. Nuptial pad in males brown or black. Flanks merging from dorsal colour to pale ventral colour, typically appearing finely spotted with white. Chin, chest and belly white, sometimes tinged cream on belly. Posterior thigh orange or deep red. Groin orange or marked with a small translucent purple patch. Axilla pale orange or cream. Ventral surfaces of hind and forelimbs orange or greyish orange; undersides of hands and feet yellowish orange.
Sexual dimorphism. Females are larger than males, being an average of 1.25 times the SVL of males ( Table 1 View TABLE 1 ). Overall body shape also differs, with females being rotund in body shape (body width is wider than head), whereas male body width starts equal to head then tapers as an inverse triangle. The sexes are otherwise similar in body proportions ( Table 1 View TABLE 1 ). Colour in preservative differs between the sexes. Male dorsum is greyish brown, whereas female dorsum is bluish grey. Male ventral surfaces in preservative are cream, whereas females tend to be white. Breeding males have obvious dark nuptial pads. Colour in life is similar between the sexes, with a few subtle differences. Females are typically brighter green than males. The thin gold line from nare to above forelimb typically more distinct in females, whereas the band below this line is typically thicker and more obvious in males. Upper lip markings (white band broken by darker bar) generally more prominent in males than females.
Tadpole description (Gosner stages 28–39). Body and tail measurements are presented in Table 2 View TABLE 2 . Total length of tadpole at Gosner Stage 39 = 32.8 mm. Body: ovoid and wider than deep with flattened abdomen; widest halfway along body ( Fig. 5 View FIGURE 5 ). Snout bluntly rounded in lateral view. Eyes dorsolateral, prominent in lateral view, with slight anterior and dorsal tilt. Nares large and narrowly spaced with slight anterior and lateral tilt; situated dorsally, near end of snout. Rim of nares raised. Spiracle lateroventral in position, opening dorsoposteriorly just below the longitudinal body axis. Inner wall and margin of spiracle opening flush with body surface. Vent tube dextral, opening dorsoposteriorly; moderately long and contiguous with ventral edge of lower fin. Tail: one-and-ahalf times as long as body with moderately shallow fins. Dorsal fin slightly arched, rising gently from back of body; highest half-way along tail. Ventral fin not arched and shallower than dorsal fin. Fins tapering to a rounded point posteriorly. Tail musculature robust, with maximum tail height greater than half of body depth. Oral disc: ventral, near front of snout. Posterior and lateral margins of oral disc fringed with papillae (see Figure 6 View FIGURE 6 ). Anterior margin of oral disc lacking papillae and flush with underside of snout. Very few submarginal papillae. Jaw sheaths darkly pigmented and shallow. Labial tooth row formula: 2(2)/3(1). Gap in second anterior tooth row broad; gap in first posterior tooth row narrow and difficult to discern without a dissecting microscope. Evidence of tooth wear common in anterior tooth rows, with first anterior tooth row incomplete in most specimens examined. Deformation and discolouration of keratinised jaw sheaths was seen in specimens collected from Kroombit Creek in December 2010, but not in specimens collected from Three Moon Creek in March 2011. Colour in life: Body brown above with areas of darker subcutaneous pigment over the braincase and gut. Darker pigment also concentrated around nares. Eyes also dark, and prominent in dorsal view ( Fig. 5 View FIGURE 5 B). Tail musculature off-white with fine grey-brown stippling, contrasting with dark brown of body in dorsal and lateral view. Darker pigment on tail musculature more obvious dorsally, occasionally forming indistinct bands, but mostly uniform or mottled. Tail fins semi-transparent, with sparse grey-brown stippling along dorsal fin. Ventral fin largely unpigmented. Gut dark in lateral view, lightening ventrally. Iris copper-gold and bisected by dark (black) lateral stripe. Ventral body surface largely unpigmented, with diffuse layer of silver-gold iridophores over ventrolateral and lateroventral body surfaces. Coiled intestine, gills and heart clearly visible through ventral body wall, especially in earlier stage tadpoles; becoming fainter in later stage (post Gosner 35) tadpoles. Colour in preservative: as in life, except for absence of silver-gold pigment over lateral body surfaces and copper-gold in iris. Early stage tadpoles: Preserved specimens younger than Gosner stage 28 were not available for study. Recently hatched tadpoles (Gosner stage 24–25) seen in the field, however, are more round-bodied and somewhat darker above compared with late stage larvae.
Comparison with tadpoles of other species: Tadpoles of L. kroombitensis sp. nov. are very similar to tadpoles of both L. pearsoniana and L. barringtonensis . Tadpoles of L. kroombitensis sp. nov. can be distinguished from L. barringtonenis on the basis of colouration, with L. barringtonensis tadpoles being significantly darker above (see Anstis 2002). Tadpoles of L. kroombitensis sp. nov. appear to be indistinguishable from those of L. pearsoniana . In the field, tadpoles of L. kroombitensis sp. nov. may be confused with early stage tadpoles of Mixophyes fasciolatus , Limnodynastes peronii and L. chloris . Early stage tadpoles of M. fasciolatus and L. peronii can be distinguished from L. kroombitensis sp. nov. on the basis of labial tooth-row formula, with the former two species possessing 3 or more anterior tooth rows from Gosner stage 25 onwards (E. Meyer, unpub. obs.; see Anstis 2002). Early stage (Gosner stage 25–26) L. chloris tadpoles differ from L. kroombitensis sp. nov. tadpoles in having eyes more lateral in position and a more cylindrical vent tube (see Anstis 2002).
Call. A short, crisp whine followed by one or two chirps, “weeak kik” or “weeak kik kik” ( Fig. 7 View FIGURE 7. A ). Call characteristics are outlined in Table 3 View TABLE 3 .
Comparison. Only likely to be confused with other members of the L. citropa species-group. Litoria kroombitensis sp. nov. is readily distinguished from the larger members of this species-group ( L. citropa , L. subglandulosa and L. daviesae ) by its smaller size (males 28–32 mm vs males 35–57 mm for latter three species), lack of prominent submandibular glands, and more uniform dorsal colouration. Distinguished from L. piperata by larger size and fairly uniform dorsal colouration ( vs heavily mottled in L. piperata ). Distinguished from L. phyllochroa and L. nudidigitus by colouration of the canthal region. Both L. phyllochroa and L. nudidigitus have a prominent white or gold canthal stripe bordered below by a sharply defined black stripe of equal thickness, and an unmarked green upper lip. In contrast, L. kroombitensis sp. nov. has a thin gold canthal stripe bordered below by a darker band or thin black line that is not so clearly defined, and prominent white along the upper lip that is typically broken below the eye by a darker bar. Also readily distinguished from all the aforementioned species by mating call.
Litoria kroombitensis sp. nov. is most similar to L. pearsoniana and L. barringtonensis , from which it can be diagnosed in a number of ways. Litoria kroombitensis sp. nov. attains a larger size than L. pearsoniana and L. barringtonensis , for both males and females ( Table 1 View TABLE 1 ). Male and female L. kroombitensis sp. nov. generally have wider heads (HW/HL) than L. pearsoniana and L. barringtonensis ( Table 1 View TABLE 1 ). Male L. kroombitensis sp. nov. generally have a lower EN/IN ratio than L. barringtonensis ( Table 1 View TABLE 1 ). In terms of colour pattern, L. kroombitensis sp. nov. typically have prominent white markings along the upper lip, broken by a dark bar or blotch below the front of the eye (particularly in males) ( Figs 2 View FIGURE 2 A–C, 3, 4). In L. pearsoniana , white on the upper lip is limited to a thin, unbroken line or is absent ( Fig. 2 View FIGURE 2 E, F). Litoria pearsoniana sometimes have a blotch or other small markings on the upper lip but this is rarely a bar from the eye to the lip, as is typical in L. kroombitensis sp. nov. The upper lip of L. barringtonensis does not have white markings or a dark bar ( Fig. 2 View FIGURE 2 D). In L. kroombitensis sp. nov., a thin gold line extends from the nare to above the forelimb, bordered below by a dark band or a thin black line. In L. barringtonensis , the gold line is not bordered below by a dark band or line. In both these species the dorsal and ventral colours merge gradually on the flank. In L. pearsoniana , the gold and dark band extends beyond the forelimb and continues in a more diffuse manner down the entire flank, resulting in the dorsal colour being fairly sharply demarcated from that of the flank. In life the tympanum of L. kroombitensis sp. nov. and L. barringtonensis are green, matching the surrounding colour and making the tympanum indistinct; whereas in L. pearsoniana the tympanum is typically brown and distinct. White gilding to the outer edges of the hindlegs and forearms is more prominent in L. kroombitensis sp. nov. than L. pearsoniana and L. barringtonensis . The calls of L. kroombitensis sp. nov. and L. pearsoniana differ in a number of ways. The whine component is shorter in L. kroombitensis sp. nov. and of faster pulse rate ( Table 3 View TABLE 3 ). The reverse is the case for the chirp component of the call, with each chirp in L. kroombitensis sp. nov. being longer and of considerably slower pulse rate than in L. pearsoniana ( Table 3 View TABLE 3 ). The faint recording obtained for L. barringtonensis allows only rudimentary comparison of the call of this species. As for L. kroombitensis sp. nov. and L. pearsoniana , the call consists of a whine followed by one or two chirps. The whine component of the individual measured was short ( 0.194 s), as for L. kroombitensis sp. nov.; whereas the chirp component was of fast pulse rate (102 pulses/s), as for L. pearsoniana . The number of pulses in chirp 1 was interesting in being 6 or 7, versus 2–4 in L. kroombitensis sp. nov. and 3–6 in L. pearsoniana . There is substantial genetic divergence among L. kroombitensis sp. nov., L. pearsoniana and L. barringtonensis , including approximately 8% 16S mtDNA sequence divergence between L. kroombitensis sp. nov. and each of L. pearsoniana and L. barringtonensis (Donnellan et al. 1999) . Litoria kroombitensis sp. nov. does not co-occur with any other member of the L. citropa species-group, being approximately 275 km north of the nearest population of L. pearsoniana and 550 km north of the nearest population of L. barringtonensis . See ‘Tadpole’ section above for comparison of L. kroombitensis sp. nov. tadpole to tadpoles of other species.
Genetics. Litoria kroombitensis sp. nov. is highly divergent from other members of the L. phyllochroa speciesgroup. It represents the ‘OTU30’ lineage presented in Donnellan et al. (1999), which is 8.1–9.7% divergent from L. pearsoniana for 16S mtDNA, 7.1–8.9% divergent from L. barringtonensis , 9.6–10.8% from L. phyllochroa , and 8.6–12.1% from L. nudidigitus . Three 16S mtDNA sequences for Litoria kroombitensis sp. nov. have been deposited in GenBank (accession numbers: KC682864 View Materials –6).
Distribution. Restricted to Kroombit Tops, south-west of Gladstone, east Australia ( Fig. 8 View FIGURE 8 ). Kroombit Tops is an isolated northern outlier to the temperate wet forests of south-east Queensland/north-east New South Wales. Based on extensive surveys since the mid 1990s, Litoria kroombitensis sp. nov. is known only from the headwaters of five streams that rise on the plateau on the eastern side of Kroombit Tops; namely Dry, Griffiths, Kroombit, Three Moon and Munholme Creeks. Records are restricted to an elevational range of about 550–900 m a.s.l. Survey effort at lower altitudes has been less intensive than at higher altitudes, but the species has not been recorded from several surveys in the lower sections of streams (e.g., Kroombit Ck). The distribution of L. kroombitensis sp. nov. lies approximately 275 km north of the nearest populations of L. pearsoniana (Mothar Mountain near Gympie, south-east Queensland) and approximately 550 km north of the nearest populations of L. barringtonensis (Washpool region, north-east New South Wales).
Habitat and habits of frogs and tadpoles. Litoria kroombitensis sp. nov. is a wet forest, stream-breeding species. It inhabits rainforest and adjoining wet sclerophyll forest ( Fig. 9 View FIGURE 9 ), where calling males and gravid females are encountered along flowing streams. Non-breeding adults and sub-adults are rarely encountered but presumably feed and shelter along the streams and in adjacent forest. Breeding activity (amplexus and/or spawning) has been recorded in all months from September through to February. Amplexus is axillary. Egg masses attributable to L.
kroombitensis sp. nov., comprising roughly 100–300 darkly pigmented eggs encapsulated in jelly, have been found wrapped around submerged twigs and branches in pools with largely static or slow-flowing water ( Fig. 10 View FIGURE 10 ). As in L. pearsoniana (E. Meyer, pers. obs.) egg masses of L. kroombitensis are covered by a fine layer of silt soon after laying. Larval L. kroombitensis sp. nov. are found in quiet pools along and adjacent to slow and intermittently flowing streams in rainforest and adjoining wet sclerophyll forest. Though only recorded during spring and summer (from September to mid-February), tadpoles may be present at other times of year as well (e.g., early autumn). Tadpoles nearing metamorphic climax and/or recently metamorphosed frogs have been recorded in November, December, January and February. Litoria kroombitensis sp. nov. tadpoles are largely benthonic, feeding on sediment (most commonly silt) at the bottom of pools. Recently-metamorphosed frogs have been recorded in summer sitting out on stream-side vegetation, including palm ( Archontophoenix cunninghamiana ) seedlings and rainforest spinach ( Elatostema reticulatum ). Breeding pools in rainforest occupied by L. kroombitensis sp. nov. larvae are free of fish except for the occasional longfin eel ( Anguilla reinhardtii). Litoria kroombitensis sp. nov. tadpoles have occasionally been recorded sympatric with larvae of Adelotus brevis (Günther, 1863) , Mixophyes fasciolatus Günther, 1864 , Litoria chloris (Boulenger,1893) and Limnodynastes peronii (Duméril and Bibron, 1841) .
TABLE 1. Morphology data. Morphology codes are: SVL: snout to vent length, HLL: hindlimb (tibiofibula) length, FLL: forelimb (radioulna) length, HW: head width, HL: head length, EN: eye to nare distance, IN: inter-nare distance, ED: eye diameter, 3 DW: third finger disc width. Sample sizes are: L. kroombitensis sp. nov. 15 males, 5 females; L. pearsoniana 21 males, 15 females; L. barringtonensis 23 males, 8 females.
| L. kroombitensis sp. nov. | L. pearsoniana | L. barringtonensis | ||
|---|---|---|---|---|
| Trait | Sex | mean range | mean range | mean range |
| SVL (mm) | M | 30.0 28.0–32.1 | 27.9 25.2–29.5 | 27.6 24.1–29.2 |
| SVL (mm) | F | 37.7 31.7–43.5 | 33.0 30.1–36.7 | 33.8 30.8–36.8 |
| HLL (mm) | M | 15.4 14.3–17.5 | 14.2 13.2–15.2 | 14.4 12.6–15.7 |
| HLL (mm) | F | 19.0 17.0–21.7 | 16.8 15.0–18.8 | 17.1 16.0–17.8 |
| FLL (mm) | M | 6.8 6.1–7.5 | 6.4 5.7–7.1 | 6.5 5.4–7.3 |
| FLL (mm) | F | 8.3 7.0–9.5 | 7.2 6.4–8.5 | 7.6 6.6–8.4 |
| HW (mm) | M | 10.4 9.4–11.5 | 9.5 8.9–10.1 | 9.5 8.3–10.6 |
| HW (mm) | F | 12.7 11.2–14.1 | 10.8 10.1–12.0 | 11.6 10.5–13.1 |
| HL (mm) | M | 8.2 7.6–8.7 | 7.7 7.3–8.2 | 8.0 7.0–8.7 |
| HL (mm) | F | 9.8 8.9–11.3 | 8.6 8.0–9.3 | 9.3 8.4–9.9 |
| EN (mm) | M | 2.93 2.56–3.33 | 2.79 2.54–3.06 | 2.80 2.46–3.23 |
| EN (mm) | F | 3.59 3.17–4.01 | 3.07 2.69–3.50 | 3.22 3.01–3.59 |
| IN (mm) | M | 2.06 1.80–2.24 | 1.96 1.69–2.19 | 1.81 1.45–2.07 |
| IN (mm) | F | 2.55 2.39–2.91 | 2.10 1.86–2.26 | 2.30 1.95–2.57 ...... continued on the next page |
TABLE 2. Morphometric data from Gosner stage 28 – 39 L. kroombitensis sp. nov. tadpoles. BL = body length; TAIL = tail length; TL = total length; MTH = maximum tail height; TMH = maximum height of tail musculature; IOD = interorbital distance; IND = inter-naris distance; OD = oral disc width.
| Gosner stage | N | BL (mm) | TAIL (mm) | TL(mm) | MTH (mm) | TMH (mm) | IOD (mm) | IND (mm) | OD (mm) |
|---|---|---|---|---|---|---|---|---|---|
| 39 | 1 | 12.8 | 20.0 | 32.8 | 5.8 | 2.5 | 3.3 | 1.3 | 3.2 |
| 36 | 1 | 11.3 | 14.0 | 25.3 | 5.5 | 2.7 | 2.8 | 1.3 | 2.8 |
| 35 | 1 | 10.8 | 16.7 | 27.5 | 5.3 | 2.5 | 2.7 | 1.3 | 2.8 |
| 33 | 2 | 10.0–10.3 | 16.0–16.7 | 26 | 5.3 | 2.5 | 2.7 | 1.3 | 2.7 |
| 32 | 2 | 10.0–10.5 | 12.2–15.0 | 22.7–25 | 4.8–5.3 | 2.3 | 2.5 | 1.3 | 2.7–2.8 |
| 29 | 1 | 7.8 | 11.7 | 19.5 | 4.7 | 1.7 | 2.2 | 1.2 | 2.0 |
| 28 | 4 | 7.3–8.3 | 10.8–13.3 | 18.2–21.7 | 3.3–3.5 | 1.7–1.8 | 1.7–1.8 | 0.8–1.2 | 1.8–2.0 |
TABLE 3. Call data. The call consists of a whine followed by one or more chirps. For details of call traits see Methods. DF is dominant frequency. Sample sizes are: L. kroombitensis sp. nov. 13 males, L. pearsoniana 8 males. Call data for L. barringtonensis is not presented because only a faint call from a single individual was obtained. Comments on this call are presented in the Comparison section.
| L. kroombitensis sp. nov. | L. pearsoniana | ||
|---|---|---|---|
| Trait | mean range | mean | range |
| Call duration (s) | 0.524 0.431–0.704 | 0.797 | 0.542–0.974 |
| Whine duration (s) | 0.195 0.160–0.229 | 0.267 | 0.174–0.369 |
| Number of pulses in whine | 115 89–146 | 113 | 81–138 |
| Pulse rate of whine (pulses/s) | 586 500–656 | 433 | 339–512 |
| Dominant freq. of whine (kHz) | 2.84 2.28–3.18 | 2.82 | 2.30–3.30 |
| Lower DF peak of whine (kHz) | 2.31 2.07–2.56 | 2.49 | 2.29–2.79 |
| Higher DF peak of whine (kHz) | 2.93 2.77–3.18 | 2.97 | 2.63–3.30 |
| Number of chirps | 1 1–2 | 2 | 1–3 |
| Dominant freq. of chirps (kHz) | 2.08 1.89–2.22 | 2.28 | 2.07–2.59 |
| Duration of chirp 1 (s) | 0.075 0.054–0.105 | 0.043 | 0.025–0.067 |
| Number of pulses in chirp 1 | 3 2–4 | 4 | 3–6 |
| Pulse rate of chirp 1 (pulses/s) | 41 34–48 | 100 | 82–127 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.