Lagidium viscacia (Molina, 1782)
|
publication ID |
https://doi.org/10.5281/zenodo.6585600 |
|
DOI |
https://doi.org/10.5281/zenodo.6610840 |
|
persistent identifier |
https://treatment.plazi.org/id/EA516C5C-FFAB-E71F-FF7C-F53FA2979B7A |
|
treatment provided by |
Carolina |
|
scientific name |
Lagidium viscacia |
| status |
|
5.
Common Mountain Viscacha
French: Viscache de montagne / German: Eigentliches Bergviscacha / Spanish: Vizcacha de montana
Other common names: Mountain Viscacha; Northern Mountain Viscacha ( Peru and northern Chile), Southern
Mountain Viscacha / Southern Viscacha ( Bolivia, Chile, and Argentina)
Taxonomy. Lepus viscacia Molina, 1782 ,
type locality not given. Restricted by W. H. Osgood in 1943 to “Chilean Andes; cordillera of Santiago.”
Some authors consider populations in Peru and northern Chile as a valid species (the “Northern Mountain Viscacha,” L. peruanum ), but this taxon is not accepted here; taxonomic revision is urgently needed. Monotypic.
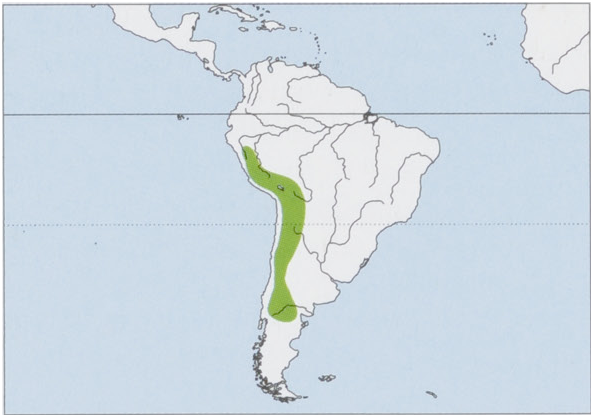
Distribution. Andes in C & S Peru, S & W Bolivia, N & C Chile, and NW & W Argentina as far S as 42° S in Chubut. View Figure
Descriptive notes. Head-body 295-464 mm, tail 215-376 mm, ear 61-82 mm, hindfoot 82-113 mm; weight 0-75-21 kg. The Common Mountain Viscacha looks like a large chinchilla but with much longer ears (larger than 65 mm on average). Tail is shorter than head-body length and usually curved dorsally. Head is rounded, short, and massive; ears are always erect. Mouth vibrissae are dark, rigid, and extremely long (150 mm) and always down directed. Fur is dense and soft, covering entire body, except naked, black plantar pads; it is comprised of dense tufts that contain a dozen or so individual hairs; sparse guard hair have slender shafts and stout tips. There is no seasonal molting pattern in adult Common Mountain Viscachas, so molting is likely continuous. Dorsal color is highly variable within and among populations within its ample distribution, but it is typically buff to dark gray; there is a striking dark dorsal stripe whose color is independent of that of the body. Venter is always paler than back, gray washed with buffy yellow. Buff or orange fur usually covers rump and upper thighs, and there may be axillary and inguinal patches of white. Dorsal surface of feet is pale, not black. Hindfeet are narrow with four digits; the fifth digit is extremely short. Forefeet have four digits. All digits terminate in short, curved claws dwarfed by enlarged toe pads. Coarse and pale hair forms a crest along the top of tail and is sharply demarcated from shorter, finer, dark hair along the bottom. Tip of tail is black or orange. Skull is narrow, with long rostrum. Interorbital region is broad (interorbital breadth larger than 16-8 mm), with weakly developed post-orbital processes offrontals that tend to be depressed between orbits. Braincase of the Common Mountain Viscacha is elevated, flat, and rounded, with no sagittal ridge and only poorly developed parietal ridging. Bullae are enlarged, with mastoid portion barely visible. Interparietal is wider than long. Jugal is broad and in contact anteriorly with lacrimal. Floor of infraorbital foramen is smooth, lacking a canal indicative of a nerve passage. Incisive foramina are long and narrow and occupy only ¢.60% of diastema length. Anterior palate is greatly restricted between premolars. Mandible has elongated and narrowed angular processes and weakly developed ridge lateral to reduced coronoid process for muscle attachment. Each cheektooth has three, distinctly curved rather than straight laminae, particularly so in M?. In the maxilla, third plate of each tooth is shorter than the other two, with that of M? forming a posteriorly pointed heel. In mandible, first plate of each tooth is reduced relative to median and posterior plates. Many different specific and subspecific names have been proposed for specimens collected in the wide geographical distribution of the Common Mountain Viscacha, but no general assessment of variation and no consistent geographical patterns have been systematically established and evaluated. Furthermore, molecular genetic distances (mtDNA sequences of cytochrome-b gene) among three geographical populations of the Common Mountain Viscacha are rather intermediate in amount of variation (4-9-7-8%) and geographically incongruent. Therefore,it is highly likely that eventual geographical units can and should be formally recognized. Nevertheless, it was not possible to do so because even the question as to whetherthis single species is composite or not remains an open issue. As a consequence, recent reviewers made no effort to delineate intraspecific taxa. Chromosome number is 64, with 126 chromosomal arms (specimens referred to L. peruanum ).
Habitat. Among rocks, rocky crevices, boulder piles, and vertical cliffs in dry and semidry areas in the high plateau of the Andes, also in pre-puna habitats, high-elevation cold desert, and Patagonian steppes. The species ranges from 700 m in Rio Negro Province, Argentina, to 4800 m in the Andes. The Common Mountain Viscacha is a poor digger with only small claws, so dens are within natural crevices or under boulder piles. Rare self-dug burrows, typically found under boulders in loose gravel or soil, probably require multiple individuals and perhaps multiple generations to construct. Presence of Common Mountain Viscachas is readily detected by distinctive and numerous fecal pellets that can be found throughout the colony, on top of rocks, or in sheltered crannies; these accumulations became solid masses preserving many organic materials, often called “rat-packs” in the literature.
Food and Feeding. The Common Mountain Viscacha is herbivorous, specializing on green leaves, flowers, fruits, stems, and bark rather than dried plant materials; it eats a wide variety of plants depending on its local environment. It does not accumulate and storage green vegetation for future use but actively forages daily. Individuals descend from their bounder-field or rocky-cliff colonies to feed on green vegetation. In southern Peru, sparse vegetative cover is mostly bunch grasses ( Stipa and Festuca ) and short shrubs (predominantly species of aster such as Lepidophyllum , Baccharis , and Senecio ). In highlands of northern Chile, Common Mountain Viscachas ate ten plant species during winter and seven during summer. Stipa bomanii was most common in diets: 20-9% in winter and 30-1% in summer. Other plants eaten only in winter were Nicotiana longibracteata, Solanaceae (12:1%) and Parastrephia quadrangularis, Asteraceae (9-4%); in turn, Festuca orthophylla (19-9%) was eaten only during summer. Twenty-two plant species (mainly grasses) made up nearly 66% of diets of Common Mountain Viscachas in north-western Patagonia in all seasons. Other species in diets were shrubs (19-7%), forbs (11%), and less than 1% of mosses, lichens, and trees. Grasses such as FE. pallescens, Poa sp. , and Stipa sp. were commonly eaten; there were preferences for Poa sp. and Stipa sp. and the shrub Schinus patagonicus ( Anacardiaceae ) in summer. Diets did not vary much seasonally but did vary relative to phenology.
Breeding. In southern Peru and northern Chile, reproductive seasons of Common Mountain Viscachas begin in late October and extend through the wet austral summer (locally called the “Bolivian winter”), with pregnant females observed in October—December and July-August. Females seem to be polyestrous, breeding after they reach c.1 kg; thus, they can have their first estrus in the year they are born. Males reach breeding condition at the same body mass or at c.7 months of age; they are capable of breeding at all seasons thereafter and for the remainder oftheirlife. A large vaginal plug is formed in the female tract following copulation. Ovulation occurs near the time of copulation and is almost always from the right ovary, with implantation nearly alwaysin the right uterine horn. Gestation is ¢.3 months, with a single, precocial young born at ¢.225 g. A postpartum estrus and pregnancy are possible, and a single female may have 2-3 litters/year. Lactation from a single pair of mammae lasts c.1 month, but young are fully furred with open eyes at birth and can probably eat plant food immediately. Life expectancy is 2-3 years for both sexes.
Activity patterns. The Common Mountain Viscachais an agile, quadrupedal bounder, like a rabbit, with propulsion provided by its long hindfeet and hindlimbs and balanced with its long tail. Pearson describes its “reckless, carefree bounds from rock to rock, or ledge to ledge” and long strides of up to 2 m, which makes its capture very difficult. It tends to use latrine areas where multiple generations produce fecal piles or rat-packs. Presence of Common Mountain Viscachas is also evident by occasional tufts of fur lodged in plants or among rocks, bushes stripped of bark, and presence of beaten pathways between outcrops within the larger home range of a colony. Individuals are very vigilant and vocalize warnings with a series of very high-pitched whistles. Both sexes whistle, and all individuals within the group will respond to calls of a single individual.
Movements, Home range and Social organization. The Common Mountain Viscacha is highly gregarious, living in colonies of 4-75 individuals, with an overall density of 0-16 ind/ha because of highly clumped distributions of occupied boulderfields. Each colony has smaller groups of usually 2-5 individuals; these groups appear to be families, although their composition does not remain constant throughout the year. Each group contains a mature male and a female (presumably parents) and 1-3 young of different ages. Oldest male offspring may be sexually mature but still live with their family group. There is no defense of individual or group territories, and entrances of different burrows may be only a few meters apart within the same colony. Individuals occupy the same burrow for long periods. Common Mountain Viscachas are diurnal, up by sunrise, often spending initial daylight hours perched on sentinel boulders where they groom their fur and soak up heat from the morning sun. In the evening, they typically feed until dark, or shortly thereafter, before returning to their shared burrows. Groups and individuals are wary, and all individuals pay attention to sharp alarm whistles of a single individual. They appear to rely most heavily on vision as the dominant sense, but different whistles may be given for aerial and terrestrial predators. Peruvian predators detected by O. P. Pearson in 1948 included the Culpeo (Pseudalopex culpaeus), the Lesser Grison (Galictis cua), the Puma (Puma concolor), the Andean Mountain Cat (Leopardus jacobitus), and various avian raptors such as the black-chested buzzard-eagle (Geranoaetus melanoleucus). In northern Chile, remains of the Common Mountain Viscacha were found in feces of the Colocolo (Leopardus colocolo) and the Andean Mountain Cat. In central Chile and Argentina, the main predator is the Culpeo. The Common Mountain Viscacha overlaps with grazing or browsing artiodactyls, such as the Vicuna (Vicugna vicugna), the Guanaco (Lama guanicoe), huemuls (Hippocamelus sp.), and several species of small rodents, predominantly the sigmodontine genera Auliscomys, Chroeomys, Chinchillula, Phyllotis, and Punomys. Common Mountain Viscachas typically host roundworms, tapeworms (Cittotaenmia), and ectoparasites (e.g. lice genera Gryopus and Philandesia). Use of communal dust baths, which protect insulated properties of fur, and bristle-like combs on hind toes help dislodge troublesome parasites.
Status and Conservation. Classified as Least Concern on The IUCN Red List. The “Northern Mountain Viscacha” ( L. peruanum ) is treated by IUCN as a distinct species and classified as Least Concern. The Common Mountain Viscacha is locally abundant at many sites; however,it has become rare or extinct in many places where it was historically numerous,particularly near towns or regions with heavy human activities. It has been locally hunted forits meat and skin since pre-Columbian times. In Chile, hunting laws have protected the Common Mountain Viscacha since 1929, and it is classified as vulnerable or in critical danger in some regions of northern Chile. In Argentina,it is classified as vulnerable. In La Paz, Bolivia, local populations currently survive in small, isolated dry patches; one occupied patch decreased 74% in area between 1999 and 2007. If a proposed urban expansion plan for La Paz is approved, ¢.75% of remaining habitat could be lost in a short time, compromising the future viability of the Common Mountain Viscacha in the area.
Bibliography. Anderson (1997), Barquez et al. (2006), Cabrera (1961), Cabrera & Yepes (1960), Canevari & Vaccaro (2007), Cortés et al. (2002), Crespo (1963), Dunnum et al. (2008), Eisenberg (1974), Galende & Raffaele (2012), George & Weir (1974), Glade (1988), Iriarte (2008), Kleiman (1974), Kleiman et al. (1979), Ledesma et al. (2009), Mann (1978), Morgan & Alvarez (2013), Mufoz-Pedreros & Gil (2009), Osgood (1943), Parera (2002), Pearson (1948, 1949), Redford & Eisenberg (1992), Spotorno & Patton (2015), Spotorno et al. (2004), Tarifa, Aparicio & Yeensens (2007), Tarifa, Fonturbel et al. (2004), Thomas (1921a), Walker, E.P. (1968), Walker, R.S. et al. (2008), Weir (1974), Weir & Rowlands (1974), Woods & Kilpatrick (2005).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.