Dasyuroides byrnei, Spencer, 1896
|
publication ID |
https://doi.org/10.5281/zenodo.6608102 |
|
DOI |
https://doi.org/10.5281/zenodo.6602735 |
|
persistent identifier |
https://treatment.plazi.org/id/EA7087C1-FFAA-2440-FA04-F7A50883000F |
|
treatment provided by |
Felipe |
|
scientific name |
Dasyuroides byrnei |
| status |
|
3. View On
Kowarl
Dasyuroides byrnei View in CoL
French: Kowari / German: Kowari / Spanish: Kowari
Other common names: Brush-tailed Marsupial Rat, Bushy-tailed Marsupial Rat
Taxonomy. Dasyuroides byrne: Spencer, 1896 ,
Charlotte Waters, Northern Territory, Australia.
Described by W. B. Spencer, Dasyuroides was for some time included in the genus Dasycercus . Recent genetic (mtDNA and nDNA) research has confidently placed Dasyuroides as sister to Dasycercus . The subspecies are plausibly separated by the Diamantina River. Two subspecies recognized.
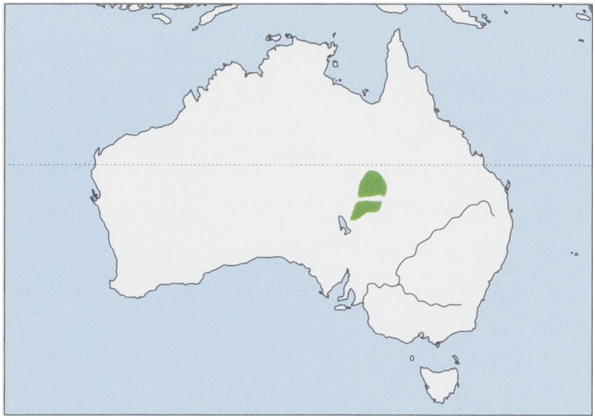
Subspecies and Distribution.
D.b.byrne:Spencer,1896—Australia,SENorthernTerritoryandSWQueensland,NWofDiamantinaRiver.
D. b. pallidior Thomas, 1906 — Australia, NE South Australia S to Cooper Creek. View Figure
Descriptive notes. Head-body 14-18 cm (males) and 13:5-16 cm (females), tail 11-16 cm (males and females); weight 85-175 g (males) and 70-140 g (females). There is moderate sexual dimorphism for size. The Kowari can be distinguished from the Brushtailed Mulgara (D. blythi) by a bushier terminal brush of black hairs onits tail and from the Crest-tailed Mulgara ( D. cristicauda ) by a brush, as opposed to a crest, of black hairs on its tail. The Kowari has four toes on each hindfoot; there are five toes in species of Dasycercus . Base oftail is never fattened in Kowaris, whereas it is typically fattened in the Brush-tailed Mulgara and very occasionally fattened in the Crest-tailed Mulgara.
Habitat. Gibber plains (rocky, pebble-littered desert pavement) between braided river channels and sand dunes where there is less than 25% shrub ground cover. Kowaris burrow mostly in small patches of sand islands on gibber plains between gray sediments of river. Presence of a low proportion of hard depressions appears to be an important feature of areas supporting Kowaris; hard depressions are relatively impermeable low points on the gibber pavement where water collects. Depending on the duration, standing water will encourage algal growth on the gibbers, which leaves a characteristic black stain. Sand mounds can form in or on the edge of such hard depressions, presumably because wind-blown soil is trapped by the water, accumulating to the point where plants can grow and act as traps for further wind-blown soil deposition. Sand Spread habitat, where an often-thin but consistent layer of soil covers large areas of gibber without forming distinct sand mounds, provides a base for plant growth but insufficient depth for large Kowari burrows. Kowaris have rarely been captured in areas dominated by large (greater than 5 cm), uneven gibbers; presumably, such habitat is too rough for them to move about quickly, and detecting and escaping stalking predators may be more difficult.
Food and Feeding. The Kowari hunts during the night. It is an opportunistic and competent predator known to hunt other native vertebrates, including the Australian Long-haired Rat ( Rattus villosissimus ). Fecal analysis, however, suggests that the majority ofits natural food items are small insects and other arthropods. Kowaris have been studied in captivity; analysis of prey-catching sequences with mice showed a temporal pattern based on frequency of occurrence of individual movements with sequences determined broadly by characteristics of prey. With small prey, such sequences were highly directional, but with larger prey, they were composed of more movements that formed alternative pathways or loops leading to a killing bite. Orientation of a killing bite of a Kowari is imprecise, and although directed anteriorly,it is not always aimed at the optimal killing region.
Breeding. Breeding in Kowaris occurs in May-December, with females carrying up to six pouch young. Local populations appear to breed independently of each other. A second, late-season breeding can occur when prey is abundant. Kowaris have an average life expectancy ofslightly more than one year, but individuals have been recorded living for longer than two years in the wild. In captivity, female Kowaris raise their young in the presence of a male and other adult females. Gestation is 35 days; young are firmly attached to a teat for c.56 days; their eyes open after c.74 days; play-fighting begins at ¢.86 days; running play and a switch to solid food start at ¢.95 days; and sexual maturity is reached at 10-11 months. Nest-building behavior of Kowaris normally starts before parturition and becomes more intense afterward. In captivity, females carried wood shavings or paperin their mouths, dragging them into the nest-box and forming a hemispherical or spherical nest. About two days after parturition, female Kowaris became active again without being disturbed by the presence of the young. During the first month of carrying pouch young, females exhibited a few obvious behavioral changes; they licked the pouch (teat area and young) and repeatedly pushed young from one side to the other with the muzzle. Females also repeatedly marked the substrate or dead mice (prey) by chestand chin-rubbing. Indeed, there was an increased frequency of sternalor chin-rubbing, and females with pouch young had an enlarged sternal gland. During later stages of infant development, some young were bruised on their heads and dorsal regions by their mother’s chinor chest-rubbing bouts. Some females with pouch young also became more aggressive toward conspecifics and humans, defending an area of 0-3-0-6 m around their nest-box. When the young became larger, they began to dangle out of the pouch and were dragged over the ground. To prevent this, females began to walk in an increasingly stiff-legged gait, with their young flexing their bodies and grabbing each other (and their mother’s legs) with their forepaws. Some days before the young first emerged from the pouch, females again became conspicuously calm, leaving their nests only rarely. During thefirst days after the young emerged from the pouch, mothers left the nest-box only for short periods, always with their young reattached. After this time, they left the young behind more often.
Activity patterns. Little is known about specific activity patterns of the Kowari. It typically remains in its burrow during daylight hours, only emerging to sun-bathe at the burrow entrance during cold,still winter days.
Movements, Home range and Social organization. Little is known about the social behavior of Kowaris. They live a solitary existence except during a brief, locally synchronized mating period and a postnatal period of less than three months, followed by dispersal of young from burrows. Local population eruptions have been recorded around Birdsville from the early 1960s, Sandringham Station in the late 1960s until 1979, from north of Birdsville in the 1980s, and south of the Konchera Sand Dune in the 1990s. Higher densities of Kowaris apparently only occur when seasonal conditions have been favorable.
Status and Conservation. Classified as Vulnerable on The IUCN Red List. Listed as Vulnerable in Australia. Numbers of Kowaris have diminished, particularly in areas where livestock has been overstocked around waterholes and bores. Kowaris have disappeared from Killalpaninna, south of Cooper Creek, and from Charlotte Waters, the type locality. Subfossil deposits in the Flinders Ranges and western New South Wales suggest the distribution of the Kowari may have been affected by increasing aridity in Australia. Parts of the habitat of the Kowari are now conserved in Diamantina and Astrebla Downs national parks in Queensland. Effects of introduced predators on the Kowari are unknown, but global warming has the potential to pose a threat. One modeling study predicted a severe contraction in distribution of the Kowari. A lack of captures of Kowaris and recent signs in some areas indicate that populations have declined and suffered local extinctions throughout the known distribution in South Australia since the 1990s. This may be a consequence of lower than average rainfall since 2002 combined with the impact of herbivores in decreasing suitability of sand mounds for shelter and lowering availability of prey items of Kowaris. Capacity of Kowaris to repopulate areas is dependent on exceptional periods of consecutive useful rains and appropriate management of suitable adjacent areas of habitat. Together,this would enable gradual dispersal of individuals when populations become saturated. In contrast, the commonplace and careless placement of stock water and heavy stocking of flooded areas adjacent to good Kowari habitat following heavy rains, likely reduce any given population of Kowaris to critically low levels.
Bibliography. Aslin (1974), Canty & Brandle (2008), Hutson (1975), Lim (1992, 1998, 2008), Mei3ner & Ganslof3er (1985), Spencer (1896¢), Westerman et al. (2007).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Metatheria |
|
Order |
|
|
Family |
|
|
Genus |
Dasyuroides byrnei
| Russell A. Mittermeier & Don E. Wilson 2015 |
Dasyuroides byrne:
| Spencer 1896 |