Manis pentadactyla, Linnaeus, 1758
|
publication ID |
https://doi.org/10.5281/zenodo.5720458 |
|
DOI |
https://doi.org/10.5281/zenodo.5720466 |
|
persistent identifier |
https://treatment.plazi.org/id/EC7D87A1-FFF3-FF8E-E27A-F972CC61F9BD |
|
treatment provided by |
Conny |
|
scientific name |
Manis pentadactyla |
| status |
|
2. View On
Short-tailed Pangolin
Manis pentadactyla View in CoL
French: Pangolin a queue courte / German: China-Schuppentier / Spanish: Pangolin colicorto
Other common names: Chinese Pangolin
Taxonomy. Manis pentadactyla Linnaeus, 1758 View in CoL ,
Taiwan.
Three subspecies recognized.
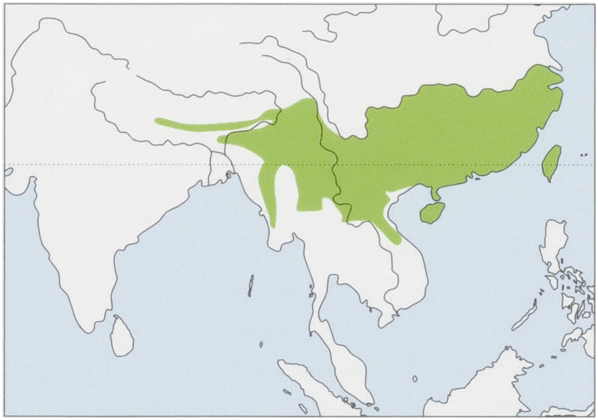
Subspecies and Distribution.
M.p.pentadactylaLinnaeus,1758—TaiwanI.
M. p. pusilla].A. Allen, 1906 — Hainan I. View Figure
Descriptive notes. Head-body 40-58 cm, tail 25-38 cm; weight 2.5-7 kg. A mediumsized pangolin, with pronounced ear pinna and dark, rounded scales contrasting with bright, yellowish-gray skin. One pair of mammae. Males larger than females. Head pearshaped and muzzle relatively short. Nose pad color similar to or slightly darker than the bright, yellowish-gray skin. Eyes small and iris dark. Scaled armor covering the upper face and the whole body except the belly and inner side of legs. Hairs bright, very thin and relatively long (longer than 0-3 cm); sparsely distributed on naked skin surfaces. Hairs project between scales. 15-17 dorsal scale rows. Width of scales of the distal part of the body similar to that of first rows of post-scapular scales; scales uniformly colored, from dark brown to dark gray. Yellowish orange scales may appear on certain regions of body, individuals then looking bicolor. Scales V-shaped, with smooth contour, even in juveniles. Tail length shorter than head and body. On tail, medio-dorsal row ofscales continuous to tip. Ventral part of tail has a narrow, terminal skin pad, due to the absence of a median scale. Forelegs slightly longer than the stout hindlegs. Five long and slightly curved claws on forefeet, especially the third one; claws turn backwards during walking, directly supporting the animal’s weight. Five slightly curved claws on hindfeet, with third claw distinctly longer. Characteristic post-anal depression, not found in any other species of pangolin. Skull 7.5-10 cm long, cone-shaped, and relatively massive. Number of caudal vertebrae: 27-28. Diploid number 2n = 40.
Habitat. Occupies a wide range of habitats, including primary and secondary rainforests, coniferous, broadleaf, shrub, and bamboo forests, and grasslands. Can also be found in areas disturbed by humans, such as agricultural fields, but seems to avoid close vicinity to settlements. Suitability of habitats is positively correlated to the density of undergrowth vegetation, especially in winter. May occur at high altitudes, notably in Nepal (up to 1500 m) and Taiwan Island (up to 2000 m); found at higher elevations where co-occurring with the Sunda Pangolin (Manisjavanica).
Food and Feeding. Myrmecophagous, this species feeds mostly on terrestrial ants in summer (including Polyrhachis), and termites in winter (among which, Macrotermes and Coptotermes). Highly selective on species preyed upon, but uses all stages from eggs to adults. Active nocturnally, using its sense of smell when foraging and while digging to reach the contents of mounds. Mostly forages underleaf litter and rotten wood; digs to follow the 0-8-2 m underground tunnels made by its prey to reach their network of nests (deeper in winter). Tunnels and nests are dug using its three central, long (up to 5 cm) forefoot claws. The termites in an average-size nest can be devoured in 30 minutes. Exceptionally large nests (reaching 90 cm of diameter) are resealed once the animalis full, and revisited the next night. In summer, an individual feeds 50-100 m around its burrow for several nights, and then moves to a new site when food becomes scarce. Winter feeding behavior is unknown. Reported to be able to go 5-7 days in summer and ten days in winter without eating. During feeding, the rostral part of the tongueis rapidly inserted and withdrawn to capture prey; the same movement is used for drinking. The lingual system is dramatically adapted to preying on ants and termites. The tongue is covered with colorless, viscous and alkaline (pH = 9-10) mucus, secreted by large salivary glands located in the pharyngeal and cervical areas and extending almost to the shoulders. With its sensory terminations and a pair of terminal, ovoid bodies with highly cornified and innervated epithelium, the tongue is very sensitive to touch. The thin, flat mandibles and weak temporo-mandibular joints seriously limitjaw movements. The symphyseal area of the mandibles forms a flat surface where the tongue can slip; extrinsic lingual muscles are attached on ridges on the insides of the mandible. During feeding, a fold of skin (plica alaris) closes the nasal passage to prevent the escape of prey. The tongue reaches c. 15 cm long and 1 cm wide. It is attached behind the larynx and trachea to a characteristically modified xiphisternum (xiphoid process). The latter is relatively shorter than in African species, and ends in an enlarged spade-shaped plate. The first third of the tongue is free and partly folded in the cervical region, and protruded when foraging. There are two stomach compartments, a storage chamber (about four-fifths of total volume) and a smaller chamber with thick muscular walls and many rugae on its inner side; the latter acts as a grinding compartment. The stomach of a 4-5 kg individual contained c.0-5 kg of food.
Breeding. Males have testes in a fold of skin located in the groin (i.e. not descended into a scrotum). Female maturity is reached in the first year, but mating seems to occur later in the year (late autumn); older females mate in late summer. Females mate outside their burrow with a single male during a 3-5 days period. Tails of male and female are entwined when copulating; each copulation lasts 3-5 min. The embryo develops in one of the uterine horns. The gestation period is poorly known, but lasts more than two months; the placenta is diffused and non-deciduate. A single young is born. Births have been reported from November to February. At birth, the young weigh 70-100 g and measure 15-21 cm. Newborns have eyes open,soft scales, and can crawl immediately; hairs protrude between scales around one week after birth. The offspring spends the winter with its mother in the burrow, where it is suckled and later provided live prey from the termite mounds sharing their den. Mother and young occupy a rearing chamber halfway along the burrow’s tunnel, thermally isolated by weeds and closed off from the main termite mound. Lactation is reported to stop after around three months. The young emerges from the burrow in spring, and is generally carried on mother’s tail. Although a stray young is generally not retrieved by its mother, an orphan may be adopted by another female. When sleeping or disturbed,it is protected by the mother’stail curled on the belly.
Activity patterns. Solitary, mostly nocturnal, and terrestrial. A poorly known species. Digs its own burrows while foraging, using its forefoot claws and inserting its tail into the ground for support; loose soil is expelled using foreand hindfeet while backing up from the burrow. Excavation rate is 2-3 m/h. Burrows are mainly found on 20-60° slopes with south-facing entrance and dense vegetation cover. There is a single entrance of 15-20 cm in diameter, which then widens into larger chambers reaching 2 m in diameter; entrance is frequently hidden or blocked with dirt. Winter is spent in a 3-4 m deep burrow, occupied by a single adult or a mother and a young. Whether the Short-tailed Pangolin uses the same winter burrow or repeatedly changes is unknown. Summer burrows are shallower (15-50 cm below ground) and shorter (80-100 cm long), and have a series of walls of dirt within them. In summer, these pangolins show a wide activity range, and generally change burrows every 2-7 days. Observed active for relatively short and interspersed periods, between 17:00-19:00 h and 22:00-02:00 h. Main walk is quadrupedal, with tail in contact with ground. Walk is usually slow, but they can move quickly using a bipedal stance on hindlegs and tail as a balance. Able swimmer and climber. Caterpillar-like walk is used to climb large trunks or branches, with prehensile tail curled up as support. Body temperature reaches 33-34-5°C. Body has a fat layer c. 1 cm thick that may contribute to insulation from external temperatures. Metabolic rate is much higher than in larger species of pangolins; it is increased to maintain stable body temperature when ambient temperature is less than 25°C. Higher affinity of its hemoglobin to oxygen compared to non-burrowing mammals suggests adaptation to long periods of stay in hypoxic environment such as sealed burrows. Respiration rhythm is irregular—from 14-20 to 42-53 breaths/minute—and sometimes almost apneic. Reported to survive 1 h of constrained submersion under water. Reacts to alarming noise by putting head between hindlegs, to present the dorsal part of the scaled armor as a first defense. In case of imminent danger, rolls into a ball. Scales on sides oftail are sharp and can be used to harm enemies.
Movements, Home range and Social organization. Virtually unknown. Rarely observed in the wild due to its secretive, solitary, and nocturnal way of life. Males fight when confronted in the field. In the presence of a receptive female, captive males may fight until death, suggesting some territoriality and/or hierarchy in the wild. Emission of a hissing sound has been reported when the animalis frightened or angry. Possesses anal glands that emit a strong and musky smell, possibly used for marking or defense; the post-anal depression is also likely to play a role in olfactory communication. Digs holes 5-10 cm deep to deposit urine and feces, using its forefoot claws or rubbing with the scales ofits neck.
Status and Conservation. CITES Appendix II. The annual export quota is zero for specimens removed from the wild for trade. Classified as Endangered on The [UCN Red List because of a dramatic reduction of populations in the last 15 years, likely to continue over the next 15 years given the heavy hunting pressure driven by the Chinese traditional medicine market. Reported to have undergone a dramatic decrease in China since 2000, with a remaining population estimated at 25,100 -49,450 individuals. In some Chinese provinces, populations have decreased by 90% in less than ten years ( Guangdong and Hunan), or may have become extirpated ( Hainan, Henan, and Jiangsu). Although protected by national legislation and present in a number of protected areas throughout its range,it is heavily exploited for its meat and its supposed medicinal properties, a situation exacerbated by continuous destruction ofits habitats. Large seizures of illegally traded animals regularly occur, and much greater law enforcementis needed to limit poaching. The Short-tailed Pangolin is reported to be an easy species to hunt, given its terrestrial way oflife and ground burrows,so the hunting threat might be even greater than to the semi-arboreal Sunda Pangolin. Since the mid-1980s, a dramatic shift from local consumption towards international trade (to China) has occurred. In Vietnam, where it has become extremely rare, most hunters sell their live catches to Chinese export, with the price now exceeding US $ 95/kg.
Bibliography. Allen (1930), Aswathanarayana (2000), Chao et al. (1993), Cheng Haiyang (1986), Cheng Haiyang et al. (1986), Choudrury (2004), Corbet & Hill (1992), Duckworth, Steinmitz et al. (2008), Ellerman & Morrison-Scott (1953), Francis (2008), Heath (1992a), Ke Yayong et al. (1999), Krause & Leeson (1974), Kubota et al. (1962), Li Zhang et al. (2008), Masui (1967), McNab (2000), Newton et al. (2008), Pocock (1924), Smith (2008), Wenhui Nie et al. (2009), Wu Shenghay et al. (2007), Wu Shibao, Liu Naifa, Li Youyu & Sun Ruyong (2005a, 2009), Wu Shibao, Liu Naifa, Ma Guangzhi et al. (2003), Wu Shibao, Liu Naifa, Zhang Yingmei & Ma Guangzhi (2004), Wu Shibao, Ma Guangzhi et al. (2004).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.