Manis tricuspis, Rafinesque, 1821
|
publication ID |
https://doi.org/10.5281/zenodo.5720458 |
|
DOI |
https://doi.org/10.5281/zenodo.5720452 |
|
persistent identifier |
https://treatment.plazi.org/id/EC7D87A1-FFF6-FF85-E7EA-FA5EC25EFC27 |
|
treatment provided by |
Conny |
|
scientific name |
Manis tricuspis |
| status |
|
8. View On
Common African Pangolin
French: Petit Pangolin / German: Weilbauchschuppentier / Spanish: Pangolin arboricola
Other common names: African White-bellied Pangolin, African Tree Pangolin, Three-cusped Pangolin
Taxonomy. Manis tricuspis Rafinesque, 1821 View in CoL ,
“ Guinée ”, West Africa.
Sometimes included in the genus/subgenus Phataginus. Subspecies mabirae from Uganda described by G.M. Allen & Loveridge in 1942, sometimes considered valid. However, the important level of morphological variation within the species seems to preclude any subspecific delimitation, pending more detailed investigations. This species is considered monotypic here.
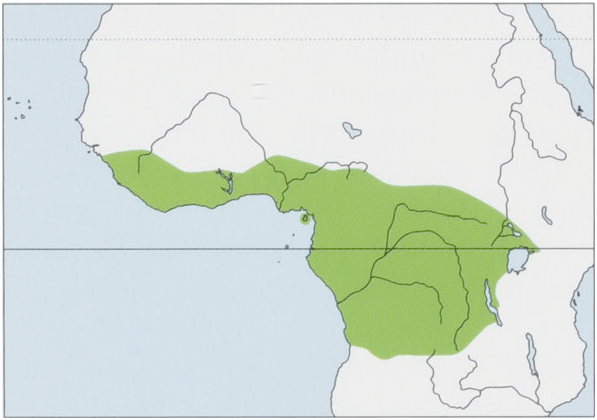
Distribution. Continuous distribution in W & C African rainforest blocks also including Dahomey Gap and Bioko I; reaches as easternand southernmost boundaries SW Kenya, extreme W Tanzania, NW Zambia, and N Angola. View Figure
Descriptive notes. Head-body 25-43 cm,tail 35-62 cm; weight 1.6-3 kg. The lightestweight pangolin, with little, three-cusped, pine cone-like scales. One pair of pectoral mammae. Males usually larger than females. Head conical and rostrum thicker than in the Long-tailed Pangolin (M. tetradactyla ), giving a more massive aspect to the nose. Nose pad color similar to the brownish skin (may reach beige). Auditory orifice without ear pinna. Eyes small but protruded; iris dark. Scaled armor with relatively small scales covering the upper face and the whole body, except the belly, the upper foreand hindfeet, and the innerside of legs. Hairs whitish on belly and throat, and brownish on legs; thin and very long (0.5-1 cm), densely imbedded. No hairs project ing between scales. 19-25 dorsal scale rows. Scales longer than wide. Width of scales of the distal part of the body similar to that of first rows of post-scapular scales; scales uniformly colored, from brownish-gray to rufous and yellowish-brown. Scales threecusped; the main, distal cusp is still visible in old adults, despite abrasion. Tail slightly longer than head and body. On tail, medio-dorsal row of scales interrupted before tip. Ventral part oftail has a large, terminal skin pad, due to the absence of two median and two lateral scales. Forelegs slightly shorter than hindlegs. Five short and curved claws on forefeet, with the third claw at least twice as long as the others; walks with wrist folded up, with claws perpendicular to ground. The five claws on the hindfeet are markedly curved and longer than in terrestrial species. Skull 6-8 cm long, V-shaped. 40-42 caudal vertebrae.
Habitat. Rainforests, savanna/forest mosaics and woodlands. Also present in humanmodified habitats, including secondary growth in oil palm groves, teak plantations, fallows, and secondary rainforest.
Food and Feeding. Myrmecophagous: mostly terrestrial termites (Microcerotermes, but also Nasutitermes), and to a lesser extent, ants (Camponotus, Cataulcus, Dorylus, Myrmecaria, Oceophylla, and Crematogaster). Most foraging is on the ground (especially in males and subadults), but arboreal mounds can also be targeted. Excellent sense of smell, and uses its protractile tongue to forage. Frequently inspects rotten branches and trunks, preys being ingested on ground or in leaf litter. Tail can be used to keep prey within a reachable range. Tree nests are broken or taken down with forefoot claws, and attacked from all sides, until ants fall down; several holes may be dug inside the nests. Nests are generally not destroyed, and thus are continuous feeding sources that are visited regularly. Foraging is mostly nocturnal, following activity cycles of prey. Females feed in a few hundred meter zone for 3-4 hours a night, whereas males forage at longer distances for 5-6 hours. The lingual system is dramatically adapted to preying on ants and termites. The tongue is covered with colorless, viscous saliva secreted by submandibular glands. With its numerous sensory terminations and a large, terminal organ similar to a pressure receptor, the tongue is very sensitive to touch. It literally acts as a “protrude and withdraw” touch organ; gustative function is expected to be minor given the small number of taste buds. The flat mandible and weak temporomandibular joints, coupled with an almost total absence of masticatory muscles, give limited movements to the jaw. The fused area of the mandibles forms a flat surface where the tongue can slip; lateral tongue muscles are attached to ridges inside the jaw. The tongue reaches 30 cm long. It is attached behind the larynx and trachea to a characteristically modified xiphisternum (xiphoid process). Xiphoid cartilages form two elongated bars that pass through the iliac fossa and then curve dorso-laterally to end as a spatula in the right crus of diaphragm. The xiphisternum provides the necessary attachments to the complex system of glossal protrudor and retractor muscles of the tongue. The hyoid bone has a different role than in other mammals, since it helps remove termites and ants from the tongue at the entrance to the esophagus. A transverse fibrous septum in the tongueitself attaches to the lingual muscles. The first one-third of the tongue is loosely attached and partly folded in the cervical region. The tongue has a cavernous tissue, also found in other mammals requiring little or no mastication, which may affect or control the level of rigidity ofits rostral part. In the stomach, a rough endoplasmic reticulum in the cytoplasm of secretory cells contributes to secreting pepsin that digests protein from prey. The epithelial lining of cardia and fundus is modified into a stratified, squamous, and keratinized epithelium to manage the chitinous load.
Breeding. Males cross several females’ ranges every night, checking estrous periods from their scenttrails. Males have testes in a fold of skin located in the groin (i.e. not descended into a scrotum). Sexual behavior is extremely elaborate. Preliminaries are simulated aggression, chest against chest, followed by the female’s submission. The female then clings to the tail of the male (like a young to its mother), and the couple moves to a tree before mating. Tails of male and female are entwined when copulating. Mean estrous cycle is nine days, with extreme individual variations (3-29 days). A single embryo develops in one of the bicornuate uterine horns. Gestation takes about 150 days; a single young is born. In captivity ( Nigeria), births were reported in November and December; young weighed c. 100 g, were born with eyes open and were active. Young stopped suckling after around four months, and weighed 750 g by seven and a half months. Maternal care is limited. Postpartum estrus happens 9-16 days after birth, after the most intense phase of maternal care and before the young pangolin starts accompanying its mother, clinging to her tail. Young is expelled by mother at next parturition, after 4-5 months. Young then goes through a period of wandering that lasts until sexual maturity, at about eight months old. Full adult size and behavior (fixed range and mating) are not reached before 15 months.
Activity patterns. Solitary, nocturnal, and semi-arboreal. Able to move from canopy to ground. Less specialized than other African forest pangolins. Mostly rests in tree hollows or piles of creepers, 10-15 m high. In Benin, shelters were mostly found in natural forest trees, preferably in velvet tamarin (Dialium guineense) and kapok (Ceiba pentandra), despite important foraging activities in human-altered zones (teak plantations). Shelters can also be found on the ground, in self-dug shallow borrows (30-40 cm deep). Terrestrial and arboreal mounds are also used as shelters. Starting period of activity is variable, from 18:00 h to after midnight. Females use a series of shelters, each for several days; when changing shelters, their range limits are also redefined. Males change shelter almost every night. Females are active for a brief 3-4 h period, and do not walk more than 600 m per night; resting sites are close to each other (less than 300 m in Gabon). Males may be active for more than ten hours and may walk 1-8 km. The period of activity is drastically shortened during the rainy season, mainly because prey become much more abundant. The Common African Pangolin has two ways of walking. The diagonal walk is the most frequently used, during which the animal moves at a mean speed of 1-1-5 km/h. A caterpillar-like walk can also be used. When moving rapidly, the tail is lifted up. Body temperature is 31-32°C during the day, below the usual mammalian range. Temperature varies from 28°C to 36°C with variation in external temperature, so thermoregulation can be considered imperfect. Thyroid gland is hypoactive in adult Common African Pangolins. Tail is long and prehensile, with a highly sensitive distal pad, used like a fifth leg. When climbing large and/or smooth trunks, the tail is curled up around the trunk and the animal spirals up, the scales, large and spread, holding onto the rough surface. The tail is also curled around branches to hold on and is used as a pivot to move between branches or trees. When in danger on the ground, the pangolins look for trees to climb, or roll into a ball, with the tip of the tail folded up on the scales of the neck, locking themselves into a defensive position. Most predation occurs on the ground, by Leopards (Panthera pardus), chimpanzees (Pan spp.), and humans. Scales are smaller and appearless protective than in other African pangolins.
Movements, Home range and Social organization. The most abundant of African forest pangolins. Adults are sedentary; ranges are notstrictly delimited, and can change from time to time. Newly weaned subadults have an amoeboid range, promoting contacts with congeners; they gradually explore farther before establishing their own range, when they weigh more than 1 kg. Males and females have overlapping home ranges of 3—4 ha and 30 ha, respectively. Males may move about 1 km each night, a small distance compared to the vastness of their ranges; in Gabon, several months of survey were needed to estimate their full ranges. Social relationships appear limited. Despite the existence of large overlapping zones in nature, territoriality seems exacerbated in males (an individual can kill another in captivity). However, olfactory markings and established hierarchy are likely to permit non-lethal regulation of contacts among males. Scent trails allow females to avoid contact with other females. In both sexes, individuals’ shelters may be very close, but never used at the same time. One male’s range may overlap with ten females’ ranges. Males tolerate females crossing their ranges, but drive away their dispersing offspring. A ventral, epidermal depression (pseudocloaca) contains the genital organs and anus, with perianal glands opening at the skin surface. The perineal glands are used in marking, sexual, and aggressive behavior, whereas the perianal glands (corresponding to epidermic bags) play a role in marking and territorial behavior. Able swimmer, using body undulation. Body almost completely above water, due to ingestion of extra air while swimming, with tail hitting the water regularly. Body diameter increased by up to 11 cm. After leaving water, emits a trumpetlike sound, emptying out extra air from nose and probably anus. In the Lama forest ( Benin), density during the dry season was estimated at 0-84 ind/km?.
Status and Conservation. CITES Appendix II. Classified as Near Threatened on The IUCN Red List because likely to have undergone a decline of 20-25% over the past 15 years, mainly due to the unsustainable level of bushmeat hunting (the species is considered close to the Threatened category). Despite its presence in a number of protected areas throughout its range, it is heavily hunted for bushmeat consumption and traditional medicine (healing/magic properties, especially ofits scales), a situation aggravated by continuous deforestation of its primary habitat in West and Central Africa. Especially appreciated for its tender dark meat, notably in Central Africa. Occupies a particular place in rainforest tribes’ mythology because of its intermediary status between fish and tetrapods. In north-eastern DR Congo, it is notably considered a “correspondent” between humans and spirits. Enforcement of protective legislation is needed in several countries to avoid local extinctions.
Bibliography. Akpona et al. (2008), Bureau et al. (1974), Doran & Allbrook (1973), Fa et al. (2006), Hatt (1934), Hoffmann (2008a), Jones (1973), Julian & Menzies (1968), Kingdon (1997), Lewis (1991), Menzies (1967), Ofusori & Caxton-Martins (2008), Ofusori, Caxton-Martins et al. (2008), Ofusori, Enaibe et al. (2008), Pages (1968, 1970, 1972a, 1972b, 1975), Pobiner et al. (2007), Rahm (1956), Sodeinde & Adedipe (1994), Soewu & Ayodele (2009), Stanley & Foley (2008), Tahiri (1966), Tahiri-Zagret (1966, 1968a, 1968b, 1969), Tahiri-Zagret & Maillet (1968).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.