Psectrosciara gonzalezae, Amorim, Dalton de Souza & Brown, Brian V., 2020
|
publication ID |
https://doi.org/10.1093/isd/ixaa001 |
|
DOI |
https://doi.org/10.5281/zenodo.3847004 |
|
persistent identifier |
https://treatment.plazi.org/id/EE454154-FFE7-BA43-3859-F88EFE31F955 |
|
treatment provided by |
Felipe |
|
scientific name |
Psectrosciara gonzalezae |
| status |
sp. nov. |
Psectrosciara gonzalezae View in CoL sp. nov.
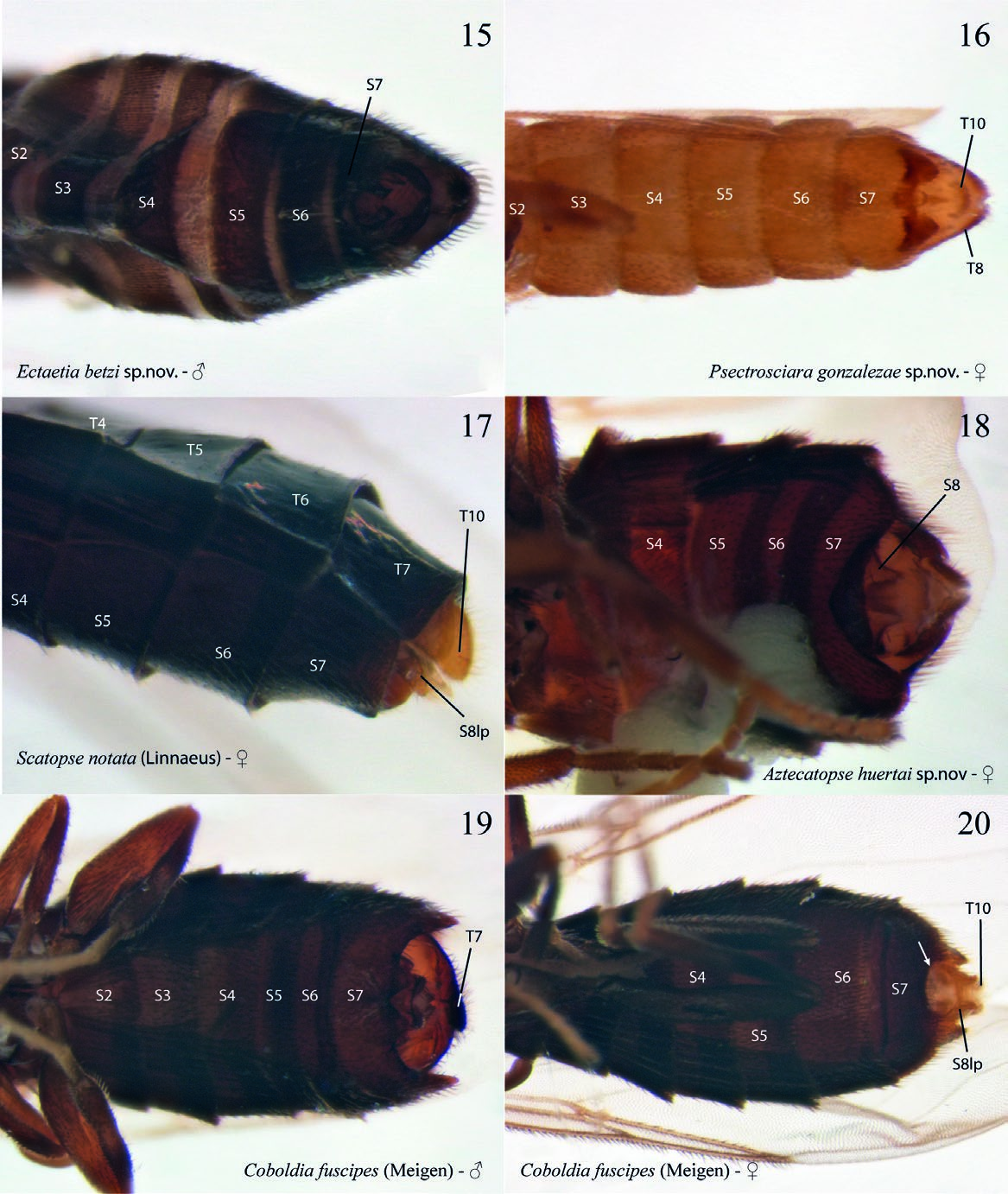
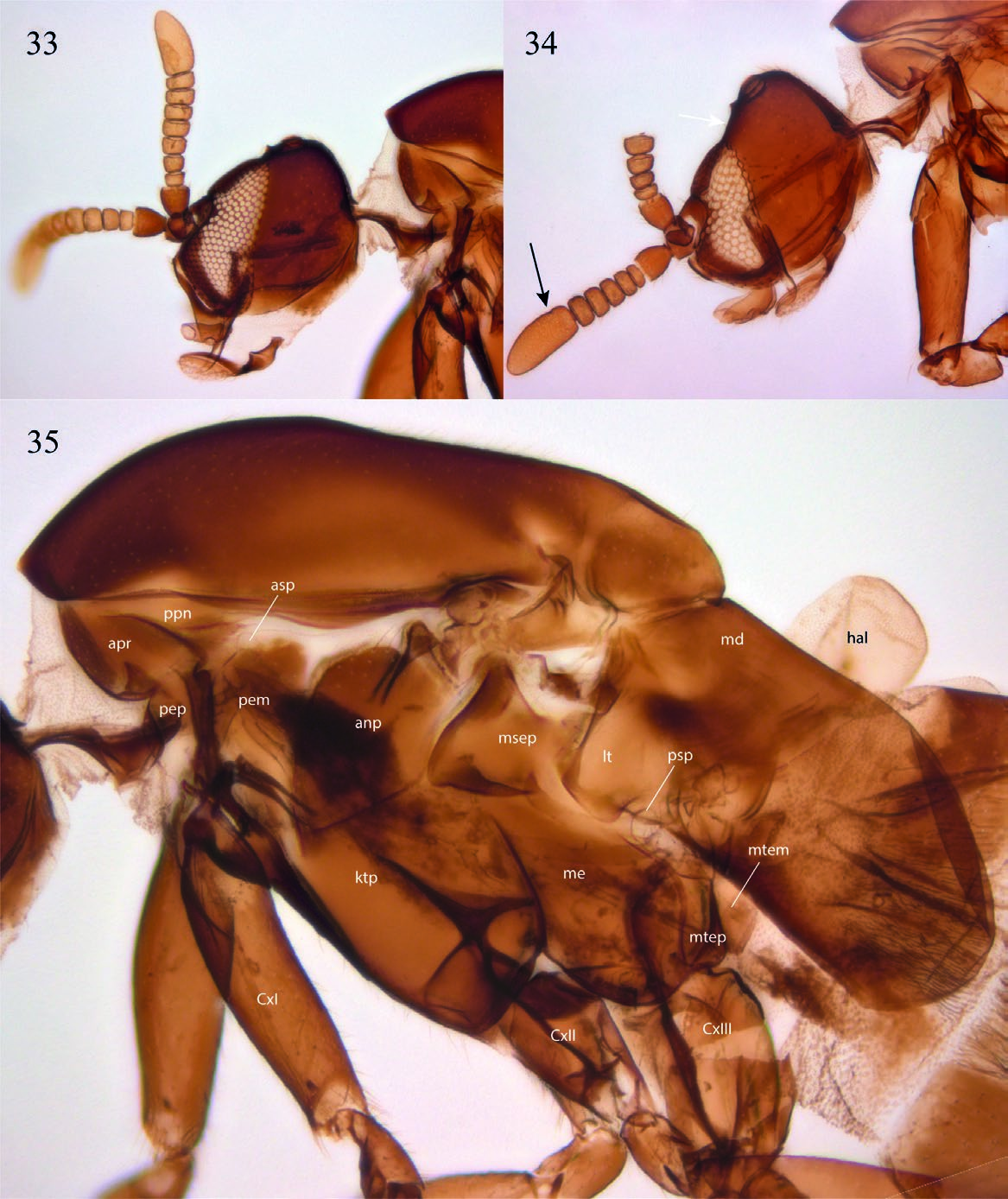
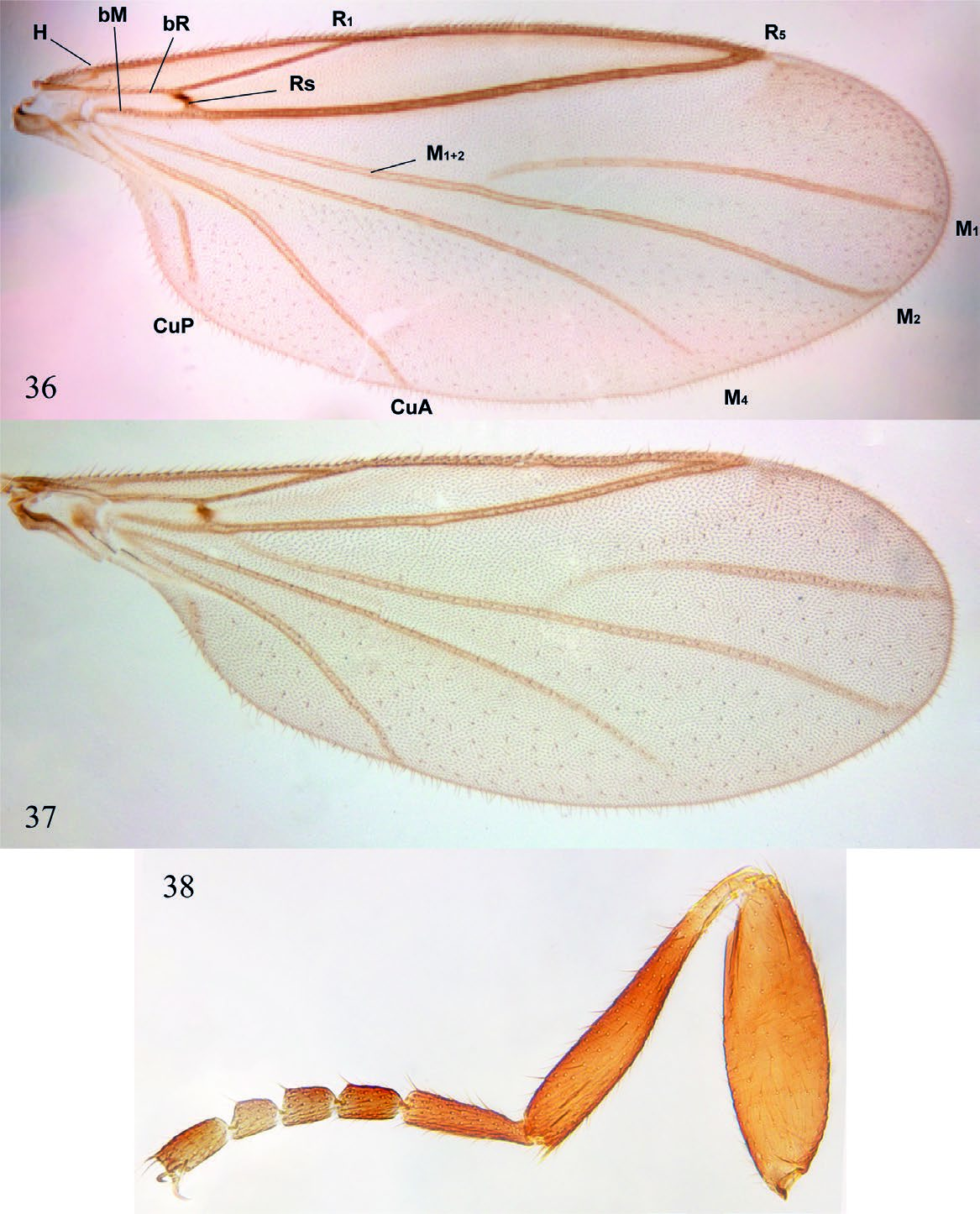
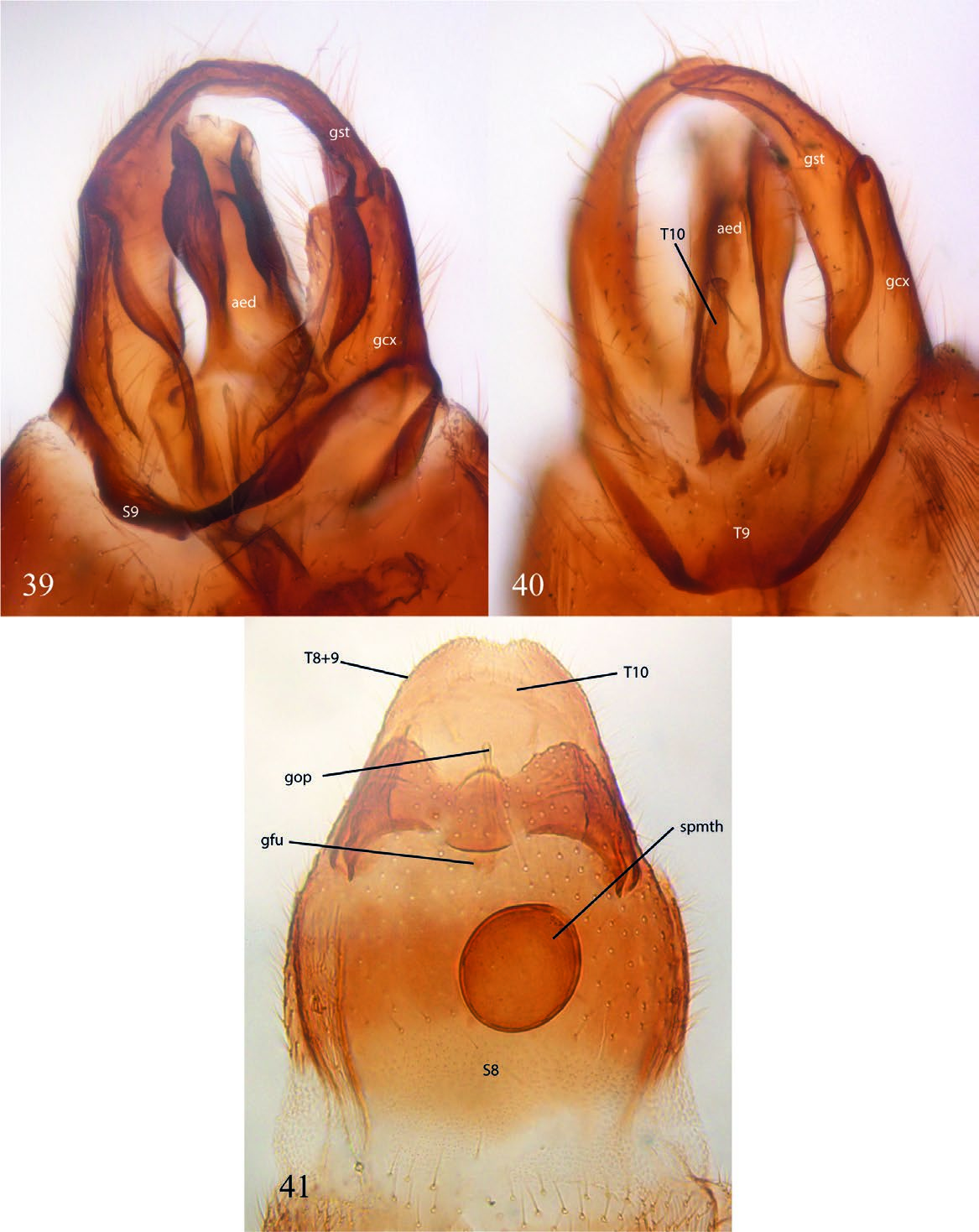
( Figs. 2 View Figs , 16 View Figs , and 33–41 View Figs View Figs View Figs )
(Zoobank LSID: urn:lsid:zoobank.org:act:)
Diagnosis. Female antenna with 7 flagellomeres. R 5 ending on distal third of wing, most of its length separate from C. No stout setae at apex of tibiae or tarsal segments. Male gonostyli typically curved inward distally; tergite 10 slender and elongated, with a small distal expansion at distal end.
Material Examined. Holotype, ♂, United States, California, Los Angeles Co., Los Angeles , Mt. Washington, 34.075° N 118.328° W, BioSCAN site 31, Malaise trap, 29 March–5 April 2014, Julian Donahue col., BioSCAN sample 15840 (on slide) ( LACM) GoogleMaps . Paratypes: 1♂, United States, California, Los Angeles County, Los Angeles, Los Feliz , 34.116°N 118.279°W, Malaise trap, 29 March–14 April 2014, BioSCAN site 6, Coll. Jeff, Aaron and Jacob Koch, BioSCAN sample 15939 GoogleMaps ; ♂, United States, California, Los Angeles Co., Los Angeles, Mt. Washington, 34.075°N 118.328°W, Malaise trap, 3–10 May 2014, BioSCAN site 31, Coll. Julian Donahue, BioSCAN sample 16009 (on slide) GoogleMaps ; 1♀, United States, California, Los Angeles Co., Los Angeles, Silverlake , 34.102°N 118.257°W, 28 June–5 July 2014, BioSCAN site 7, Coll. Joe Hogg, BioSCAN sample 16193 GoogleMaps ; 1♀, same data, Mid-City , 34.047°N 118.334°W, Malaise trap, 2–9 November 2013, BioSCAN site 12, Coll. Sharon Oxborough, BioSCAN sample 15293 GoogleMaps ; 1♀, same data, 14–21 September 2013, BioSCAN sample 15202 GoogleMaps ; 1♀, same data, Hollywood , 34.095°N 118.334°W, Malaise trap, 30 April–7 May 2014, BioSCAN site 16, Coll. Tony Hein, BioSCAN sample 15984 GoogleMaps ; 1♀, United States, California, Los Angeles Co., Los Angeles, Hollywood , 34.095°N 118.334°W, 100 m, 3–10 September 2014, BioSCAN site 16, Coll. Tony Hein, BioSCAN sample 16519 GoogleMaps ; 5♀, same data, Carthay , 34.059°N 118.369°W, Malaise trap, 1–8 April 2014, BioSCAN site 19, Coll. Teresa Dahl, BioSCAN sample 15812 GoogleMaps ; 1♀, United States, California, Los Angeles Co., Los Angeles, Carthay , 34.059°N 118.369°W, 41.1 m, 1–8 July 2014, BioSCAN site 19, Coll. Teresa Dahl BioSCAN sample 16334 GoogleMaps ; 1♀, same data, 25 February–4 March 2014, BioSCAN site 19, Coll. Teresa Dahl, BioSCAN sample 15807 GoogleMaps ; 2♀, same data, 29 October–5 November 2013, BioSCAN site 19, Coll. Teresa Dahl, BioSCAN sample 15325 GoogleMaps ; 4♀, same data, 28 October–4 November 2014, BioSCAN site 19, Coll. Teresa Dahl, BioSCAN sample 16871 GoogleMaps ; 6♀, United States, California, Los Angeles Co., Los Angeles, Carthay , 34.059°N 118.369°W, 41.1 m, 30 September–14 October 2014, BioSCAN site 19, Coll. Teresa Dahl, BioSCAN sample 16868 GoogleMaps ; 1♀, United States, California, Los Angeles Co., Los Angeles, Echo Park , 34.074°N 118.264°W, 133.5 m, 1–8 July 2014, BioSCAN site 22, Coll. Erin Johnson, BioSCAN sample 16298 GoogleMaps ; 1♂, United States, California, Los Angeles County, Los Angeles, Mt. Washington, 34.075°N 118.328°W, Malaise trap, 29 March–5 April 2014, BioSCAN site 31, Coll. Julian Donahue, BioSCAN sample 15840 GoogleMaps ; 1♀, same data, BioSCAN site 31, 31 May–7 June 2014, BioSCAN sample 16202 GoogleMaps ; 1♂, same data, 3–10 May 2014, Coll. Julian Donahue, BioSCAN sample 16009 GoogleMaps ; 1♀, United States, California, LA County, Monrovia. 34.158°N 117.999°W. Malaise trap, 1–8 September 2016, BioSCAN site 35, Coll, Brian Brown, BioSCAN sample 18375 (on slide) GoogleMaps ; 3♀, United States, California, Los Angeles County, Claremont , 34.09°N 117.711°W, Malaise trap, 1–8 June 2016, BioSCAN site 37, Coll. Kathryn Turner, BioSCAN sample 18337 GoogleMaps ; 2♀, United States, California, Los Angeles County, La Mirada , 33.914°N 118.005°W, Malaise trap, 1–8 August 2016, BioSCAN site 43, Carmen Gendusa, BioSCAN sample 18383 GoogleMaps ; 1♀, same data, 1–8 April 2016, BioSCAN site 43, Coll. Carmen Gendusa BioSCAN sample 18265 (on slide) (male paratype of BioSCAN sample 16009 and female paratypes of BioSCAN samples 18265 and 18375 at MZUSP; all other paratypes at LACM) GoogleMaps .
Description. Male. Body general color reddish brown. Body length, 3.22–3.40 mm. Head ( Fig. 33 View Figs ). Slightly longer than high; holoptic, eye-bridge large, about 8 facets wide; eye setose, ommatotrichia small; all ommatidia of similar size; three ocelli, lateral ocelli much larger than mid ocellus; frontal area between eye-bridge and ocelli almost inexistent. Occiput with fine setae distributed irregularly on occiput, a sensillum midway between ventral margin and vertex; frontal furrow marked by a line; frons between eye-bridge and base of antennae with few setae; frontoclypeus elongate, with fine setae. Labella short, setose. Maxillary palpus about as long as labella, covered with scattered setae, with an apical rounded sensory pit and a smaller pit at about mid of palpus. Postmentum laterally compressed at base, with a pair of lateral arms widening towards apex. Antenna slightly longer than head; scape slightly wider than long, pedicel subcylindrical, longer than wide; 8 flagellomeres covered with scattered fine setulae, no sensilla; flagellomeres 1–7 wider than long, flagellomere 8 club-shaped. Thorax ( Fig. 35 View Figs ). Scutum longer than wide, laterally compressed, anterior third more sclerotized that posterior two thirds; rather sparsely covered with short setae, some slightly longer setae above wing base, but no strong supraalars. Antepronotum and proepimeron setose; spiracular sclerite (dorsal half of proepimeron) small, setose, slightly longer than wide, spiracle small, confined to posterior margin of sclerite, remaining proepimeron well-developed, setose. Anepisternum with fine setae along anterior and dorsal margins, katepisternum with fine setae along anterior margin and dorsal two-thirds. Mesepimeron with 10 fine setae along dorsal margin. No meral setae, nine subspiracular setulae, metepisternum well-developed, with scattered setae. Legs. Coxae brown, femora, tibiae, and tarsi light brown. Fore coxa as long as fore femur, femora and tibiae of similar length; femora slightly swollen, fore tibia club-shaped, slightly widening towards apex. No tibial spurs, tibiae with a row of distal elongate setae. All first tarsomeres longer and wider than distal tarsomeres, no differentiated setae, spines or row of palisade setae on tarsomeres. Tarsal claws slender, curved, without dentation; empodium well-developed. Wing ( Fig. 36 View Figs ). Wing extending to beyond sixth abdominal segment, 2.43– 2.65 mm long, 0.98–1.10 mm wide. All wing cells with microtrichia, all posterior cells with scattered macrotrichia, all veins with dorsal macrotrichia, R 5 with dorsal and ventral macrotrichia; anterior veins well sclerotized, posterior veins faint. Wing length/h-R1 length, 3.42; WL/R 1 -R 5 length, 2.13; h-R 5 length/WL, 0.76. A basal anterior vein that may be a short Sc or a modified Hu; first sector of Rs oblique, base of M 1 + 2 partially fused to R 5, no r-m; basal fifth of M 1 faint, very basal portion of M 1 missing; M 1, M 2, M 4, CuA and CuP reaching wing margin, distal end of M 4 slightly beyond tip of R 5; CuA curved towards base at proximal third, reaching wing margin slightly before tip of R 1. CuP present, not reaching wing margin. Haltere light brown, with no setae. Abdomen ( Fig. 15 View Figs ). Reddish brown. Pretergite 2 present, bare. Tergite 1 rectangular, anterior margin slightly emarginated, tergites 2 to 6 slightly more slender at posterior margin; tergite 7 slightly emarginated medially. Sternite 1 unsclerotized; sternites 2 to 6 rectangular, slightly longer than wide; sternite 7 entirely divided medially by a membranous area. Spiracle 7 at membrane. Terminalia ( Figs. 39–40 View Figs ). Laterally compressed; sternite 9 fused to gonocoxites, V-shaped, slender; parameres not evident, maybe lost; gonocoxites elongate, projected medially, entirely fused laterally to tergite 8 + 9, elongate, almost reaching level of tip of aedeagus; gonostyli elongate, basal half fit at inner face of gonocoxite, distal half digitiform, curved, entirely covered with fine setae, but no spines; aedeagus well-developed, with a mesal flat ventral area distal to aedeagus opening; tergite 8 + 9 about V-shaped, with pair of spiracles 8; tergite 10 articulated to mid-posterior margin of tergite 8 + 9, elongate, with few setae on basal half, expanding only slightly at apex. Cerci absent.
Female ( Fig. 2 View Figs ). Similar to male, except for following. Body length, 2.40–2.60 mm. Head ( Fig. 34 View Figs ). Antenna with six flagellomeres, frontal area between eye-bridge and ocelli almost as long as eye-bridge. Legs ( Fig. 38 View Figs ). Thorax. Mesepimerals, 5; subspiracular, 3. Wing ( Fig. 37 View Figs ). Length, 1.22–1.45 mm; width, 0.50–0.55 mm. Wing length/h-R1 length, 3.00; WL/R1-R5 length, 2.43; h-R5 length/WL, 0.75. Abdomen ( Fig. 16 View Figs ). Spermatheca ovoid. Tergite 7 subrectangular, wider than long, posterior margin straight, spiracle 7 at membrane; sternite 7 longer than wide, partially covering terminalia ventrally, posterior margin with a sclerotized fold with a median projection, no lateral digitiform projections. Terminalia ( Fig. 41 View Figs ). Tergite 8 + 9 trapezoid, largely developed, bearing a pair of spiracles. Sternite 8 hardly sclerotized, genital furca (= sternite 9) wide, quite unsclerotized, without anterior projection. Tergite 10 slender, present as a stripe with some setae across distal margin of terminalia.
Distribution. United States, southern California.
Etymology. The specific epithet of this species name honors Lisa Gonzalez, a key member of the BioSCAN team. She was responsible for most of the logistics of the BioSCAN project and was involved and participated in the sorting process, the important effort that makes the material of the project accessible to the specialists.
Remarks. Nine species of Psectrosciara have been described to date from the Nearctic region, five of which were recorded for California. Among the Nearctic species of the genus, five belong to the group- brunnescens and four to the group- scatopsiformis ( Cook 1958, 1963). The number of species of Psectrosciara known from California is quite surprising, compared to the diversity of the genus in any other area in the world. It could be the case that these species have extremely restricted endemism or a strict association to certain plants or environments.
Of the four Nearctic species of the group- scatopsiformis, two have been recorded for California, P. californica (Cole) and P. brevipennis Cook. Psectrosciara californica ( type locality, Laguna Beach, California) is known from the region around Los Angeles. None of the species found in our samples, however, belong to the group- scatopsiformis. Cook (1958: 594) addressed in good detail the differences between males of these species from California, Arizona, and Córdoba, Mexico. The type locality of P. brevipennis Cook is El Centro, California, more to the east and very close to the border with Mexico, but it is also known from Lindsay, central California.
Of the species of the group- brunnescens, P. discata Cook ( type locality, Auburn, California) and P. forcipata Cook ( type locality, Berkeley, California) are restricted to northern California ( Cook 1958) and may not reach Los Angeles area. The type locality of P. bakeri Cook is Claremont, in Los Angeles Co. Because of all specimens of Psectrosciara from different BioSCAN sites in our samples belong only to the new species described here, it may be the case that the distribution of P. bakeri is extremely reduced, restricted to natural environments around Claremont.
Psectrosciara gonzalezae sp.nov., found in the BioSCAN samples, is clearly devoid of the stout setae at the apex of the tibiae and does not show the modified tarsal segments that characterize the groupscatopsiformis. The male tergite 8 + 9 has the typical medial posterior projection, slender and elongated, but does not have the distal rounded expansion seen in P. oregonensis Cook and in P. discata Cook ( Cook 1958) . Also, the gonostyli are clearly curved inwards distally, more like P. forcipata .
There is not much information on the biology of the genus. The holotype of P. discata was collected with a citronella bait trap, while P. stonei Cook and P. serrata were reared from cotton bolls ( Cook 1958). Our catches of Psectrosciara occurred in 16 different sampling sites (1, 7, 8, 25, 29, 30, 34, 39, 43, 52, 57, 59, 77, 90, 99, and 103).
All sequenced Psectrosciarinae specimens in the Bold Systems database are of Anapausis species.
| LACM |
Natural History Museum of Los Angeles County |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Psectrosciarinae |
|
Genus |