Simulium hasekei, Seitz & Adler & Remschak, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5040.1.7 |
|
publication LSID |
lsid:zoobank.org:pub:D60CF316-4902-4DBB-A98A-9981D2DA439C |
|
persistent identifier |
https://treatment.plazi.org/id/F249879A-1C67-FFDD-FF18-F8FCFF53DCA2 |
|
treatment provided by |
Plazi |
|
scientific name |
Simulium hasekei |
| status |
sp. nov. |
Simulium hasekei View in CoL new species
( Figs. 1–18 View FIGURES 1–8 View FIGURES 9–16 View FIGURE 17 View FIGURE 18 )
Holotype. Male with associated pupal exuviae and cocoon in 75% ethanol; abdominal tip with genitalia parts in two microvials with glycerine, left and right hind legs slide-mounted in Euparal , Austria, ponor stream at Miesbodenmoor, near Bad Mitterndorf, 47°29’38.7’’N 13°52’39.8’’E, 1408 m elevation, collected 11 July 2020, emerged 19 July 2020, leg. Christina Remschak (deposited in the ZSM). GoogleMaps
Paratypes ( Table 1). Female allotype with associated pupal exuviae and cocoon in ethanol (mandibles, laciniae, and left hind leg slide-mounted in Euparal, abdominal tip in separate microvial with glycerine; ZSM); 10 larvae, 20 pupae /exuviae (in ethanol), 3 microscope slides with larval mandibles, maxilla, hypostoma, and postgenal cleft (in Euparal; ZSM); larvae (Carnoy’s fixative, transferred to ethanol after chromosome extraction; CUAC); remaining larvae and pupae/exuviae (in ethanol; Collection G. Seitz) .
Etymology. The species is named in honor of Harald Haseke who initiated the EU Life+ project “Ausseerland”, Styria, where hydrobiological studies were carried out for long-term monitoring of potential changes. Without his help, we would never have visited this small and short ponor brook where the new species lives.
Male. Body length 3.2 mm; length of defective wing not reliably measured. Scutum black; thoracic hair golden. Stem vein and basicosta with long, brown hair. Lower part of basal fringe dark brown; upper part of basal fringe, mesepimeral tuft and abdominal hair pale brown and shining in reflected light. Legs brown with light patches; base and tip of femora and tibiae darker; recumbent pilosity brown and coppery shining in reflected light. Hind leg with basitarsus bent during emergence from pupa, not fully extended; calcipala about ½ width of apex of basitarsus; pedisulcus distinctly developed. Male genitalia: Ventral surface of gonocoxite with long brown hairs, shorter dorsally. Gonostylus at base almost ½ as wide as entire length, shorter than coxite, nearly parallel sided, distally with medially directed, subtriangular flange bearing 1 apical spinule ( Fig. 1 View FIGURES 1–8 ). Ventral plate in profile bent; in ventral view ( Fig. 2 View FIGURES 1–8 ) with body trapezoidal, posterolaterally rounded, anterior margin almost straight, posterior margin concave; lip with hairs originating from small cuticular nodules; basal arms shorter than length of body of ventral plate; in terminal view ( Fig. 3 View FIGURES 1–8 ) with body narrow and lip blunt and broad. Parameres each with 1 strong spine; dorsal plate spatulate, with flange-like collar; median sclerite elongated, slender, apically bifurcated ( Fig. 4 View FIGURES 1–8 ).
Female. Body length 3.1 mm; wing length 3.1 mm. Scutum, humeral angles, and scutellum dark brown; thoracic hair silver with golden shine. Postnotum dark brown with silver hair. Frons at narrowest width about 1/7 width of head; frons and clypeus with silver hair. Mandibles with 1 member of pair bearing 34 serrations and the other 35 serrations (15/14 outer and 19/21 inner); laciniae each with 21 retrorse teeth. Palpomere V about 1.7 times longer than palpomere IV and about 1.7 times longer than palpomere III. Sensory vesicle in lateral view elliptical, nearly 2/3 as long as palpomere III. Stem vein and basicosta with silver hair. Basal fringe silver and mesepimeral tuft silver with golden shine. Precoxal bridge complete. Legs light brown, darker on tarsi and apices of femora and tibiae; hair on tarsi light brown, silver on other segments. Hind leg with basitarsus about 6.7 times longer than wide at widest point; calcipala half as wide as apex of basitarsus; pedisulcus distinctly developed. Claws each with basal, thumblike lobe. Terminalia: Hypogynial valves almost transparent, each with few small hairs on basal half, otherwise unhaired; inner margins slightly concave ( Fig. 6 View FIGURES 1–8 ). Genital fork with stem long, well pigmented, 1.4 times longer than each comparatively long, straight arm ( Figs. 5, 6 View FIGURES 1–8 ). Arms distinctly divergent, extended posterolaterally beyond lateral plates, enclosing wide U-shaped space. Each lateral plate slightly darker and more sclerotized with barely discernible, short, blunt apodeme ( Fig. 5 View FIGURES 1–8 ). Spermatheca longer than broad, about half length of stem of genital fork; area surrounding junction with spermathecal duct unpigmented ( Figs. 5, 6 View FIGURES 1–8 ). Cercus strongly haired on both sides, in lateral view almost rectangular, broader than long, with corners rounded ( Figs. 7, 8 View FIGURES 1–8 ). Anal lobe sclerotized at edges, medioventrally extended, covered with few stout hairs compared with cercus ( Figs. 7, 8 View FIGURES 1–8 ).
Pupa and cocoon. Body length of pupa (without cocoon and gill) up to 3.9 mm, with cocoon up to 4.8 mm. Gill ( Figs. 9, 10 View FIGURES 9–16 ) of 4 initially divergent filaments directed anteriorly, slightly longer than pupa if straight and fully extended; lower pair of filaments branched inward in almost horizontal or rather diagonal plane; upper pair of filaments branched in vertical or rather diagonal plane; filaments branching at acute angle from common basal trunk, which is up to 1.5 times longer than wide. Upper trunk longer than lower trunk or with trunks of subequal length; upper and lower trunks of same thickness ( Figs. 9, 10 View FIGURES 9–16 ). Filaments with transverse furrows; head plate and anterior thoracic dorsum densely covered with oval (10 µm x 5 µm) or circular, smooth microtubercles (diameter up to 7.5 µm) ( Fig. 10 View FIGURES 9–16 ); thoracic trichomes unbranched with curved apices, up to 140 µm long. Lower part of frontoclypeal shield with 1 short, centered seta per side (often broken). Terminal spines small, pointed. Cocoon slipper shaped, weakly woven and transparent, with small particles entangled in weave; rim thickened, with anteromedial projection partially thickened by woven strands ( Figs. 11, 12 View FIGURES 9–16 ).
Larva. Length of last instar up to 6.3 mm. Ground colour of body whitish yellow; dorsally with more or less distinctly pigmented brownish areas. Head yellowish brown ( Figs. 13, 14 View FIGURES 9–16 ), with positive, brown head spots diffuse around their borders; frontoclypeal apotome paler than postgenae; posteromedial head spot in form of elongated isosceles triangle ( Fig. 14 View FIGURES 9–16 ). Postgenal cleft longer than wide, up to 210 µm long and up to 160 µm wide, rounded or tapered, extended ½ distance or more to hypostomal groove ( Fig. 13 View FIGURES 9–16 ); subesophageal ganglion unpigmented. Hypostoma ( Fig. 16 View FIGURES 9–16 ) with median tooth extended slightly beyond 2 lateral teeth that each arise from broad base; 3 small sublateral teeth per side. Paralateral teeth composed of 2 strong teeth on each side followed by 2 to 4 lateral serrations per side, sometimes reduced to 1 serration; 3 long sublateral hypostomal setae followed by 2 very short setae (often missing), arranged one behind another per side, decreasing in length, with anteriormost seta about 110 µm long, 2.5 µm thick, deeply divided. Antenna longer than stem of labral fan, up to 0.55 mm long, with proximal article more brownish, lower part of medial article pale brown and upper part translucent and free of pigment, distal article pale brown; proportion of articles proximal to distal (excluding apical sensillum) 1.4:1.5:1.0. Maxilla 150 µm long, at base 40 µm wide. Mandible ( Fig. 15 View FIGURES 9–16 ) with typically developed apical and preapical teeth, followed by 8 spinous comb teeth; inner preapical ridge of mandible with 1 sensillum and up to 5 small acute-angled serrations. Labral fan with up to 50 primary rays. Abdomen with conical ventral tubercle on each side of last segment. Anal sclerite X-shaped, with sclerotized areas alongside arms and between 2 halves; anterodorsal arms shorter than posterodorsal arms. Cuticle between arms with about 20 bent hairs, up to 40 µm long; no rectal scales. Rectal papillae with 3 compound lobes, each with 18 to 20 lobules. Posterior proleg with up to 11 hooks in 69 rows.
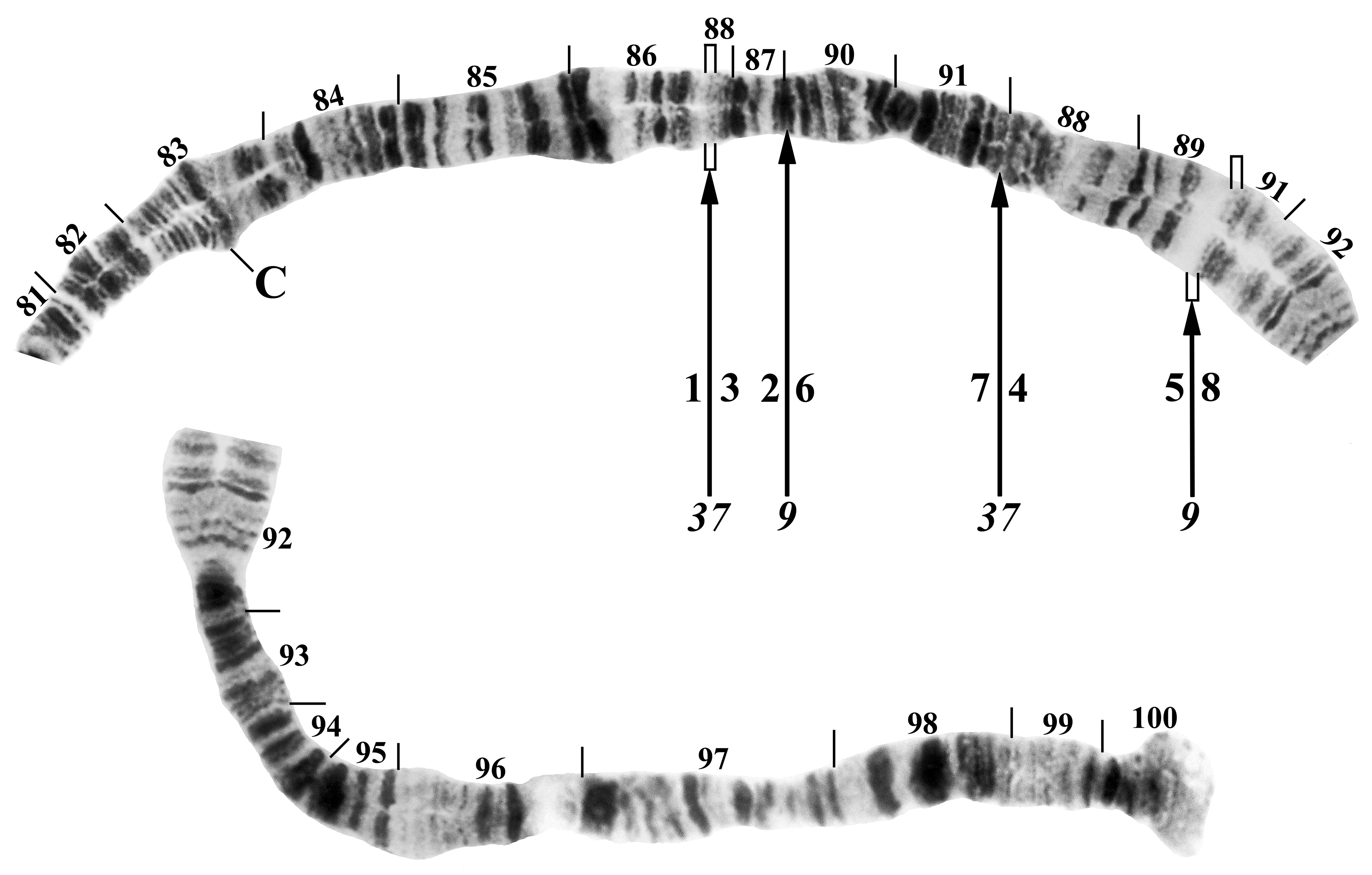
Polytene chromosomes. Chromosomal homologues were tightly paired. A chromocenter, ectopic pairing, and B chromosomes were absent. Compared with the standard banding sequence for the S. vernum group ( Brockhouse 1985), the new species differed by 1 fixed inversion in chromosome arm IL (IL-2) and the following 3 inversions tentatively regarded as fixed in chromosome arm IIIL: IIIL-1, IIIL-9, IIIL-37 ( Fig. 17 View FIGURE 17 ). The IIIL-1, 9, 37 sequence represents the DD sequence of Hunter (2002), who did not name the IIIL-37 inversion that produced the D sequence. We present the caveat that the analysis is based on only 8 larvae (all females) and, therefore, fixation of the IIIL sequence is a hypothesis requiring additional specimens for testing. The stepwise reversion of the IIIL D sequence to the standard sequence of Brockhouse (1985) is as follows, where the numbers 1–8 define the chromosomal segments of Hunter (2002) and correspond with the numbers below the chromosome in Fig. 17 View FIGURE 17 , and each pair of slashes represents inversion breakpoints:
IIIL-1, 9, 37 sequence: 1 / 3.2 6.7 / 4.5 8 (= D sequence of Hunter 2002)
IIIL-1, 9 sequence: 1 7.6 / 2.3 4.5 / 8 (= A sequence of Hunter 2002)
IIIL-1 sequence: 1 / 7.6 5.4 3.2 / 8
standard sequence: 1 2.3 4.5 6.7 8
The implication in this stepwise sequence is that IIIL-1 was the first inversion to have appeared, followed by IIIL-9 and lastly IIIL-37.
The pericentric IIIP-1 inversion of Hunter (1987) was absent ( Fig. 17 View FIGURE 17 ). Autosomal polymorphisms were restricted to one inversion, expressed heterozygously, in IS (specimen collected on 30 June 2020), with breakpoints approximately in sections 8 and 11. Sex chromosomes could not be assessed because no male larvae provided chromosomes of adequate quality for analysis.
Diagnosis and taxonomic remarks. Males of Simulium hasekei n. sp. can be distinguished from those of other European members of the Simulium vernum group by the long spatulate dorsal plate and the broad trapezoidal ventral plate with small cuticular nodules on the lip and short basal arms. In comparison with other alpine species in the Simulium vernum group in Europe ( Rivosecchi 1978, fig. 68), the width to height ratio of the ventral plate resembles that of Simulium dolomitense (Rivosecchi) . For females, the almost rectangular cercus with rounded corners and few strong hairs on the ventrally extended anal lobe, as well as the genital fork with long, straight arms are striking. The pupa is characterized by the weakly woven cocoon with a mostly well-developed anterodorsal projection, and the gill with the lower pair of filaments in an almost horizontal or rather diagonal plane and the upper pair of filaments branched in a vertical or rather diagonal plane. Larvae can be distinguished by the shape of the postgenal cleft and by the hypostoma with its strong paralateral teeth, as well as by the high number of fan rays that is not seen in other alpine species of the Simulium vernum group reported by Rivosecchi (1978) or by Seitz & Adler (2017) for Simulium arminii . In comparison with other members of this group, a well-developed larva is hard to mistake. In contrast, the pupa resembles that of Simulium crenobium (Knoz) , based on the lower pair of filaments in an almost horizontal plane; the larvae and adults of S. crenobium , however, differ markedly from those of S. hasekei n. sp. ( Ilmonen et al. 2009). The broad ventral plate and other life-stage characters distinguish S. hasekei n. sp. from species distributed beyond Central Europe, such as S. bicorne Dorogostaisky, Rubtsov & Vlasenko , S. craigi Adler & Currie , and S. fontinale Radzivilovskaya.
Chromosomally, the inversion profile of the new species provides insights into its nearest relatives. Although the IL-2 inversion is taxonomically widespread in the S. vernum group and of little phylogenetic utility ( Adler et al. 2020a), the inversions in IIIL are more restricted and are shared with the following studied members of the S. vernum group in the Holarctic Region: S. aestivum Davies, Peterson & Wood and S. bavaricum Seitz & Adler , which share IIIL-1 with S. hasekei n. sp., and S. bicorne , S. craigi , and S. fontinale , which share IIIL-1, IIIL-9, and IIIL-37 ( Hunter 2002, Seitz & Adler 2009, Adler et al. 2004). IIIL-37, however, is not fixed in S. bicorne , S. craigi , or S. fontinale , although we tentatively take it to be fixed in S. hasekei n. sp. Regardless of fixation, S. hasekei n. sp. is most closely related to S. bicorne , S. craigi , and S. fontinale , all four species sharing IIIL-1, 9 ,37 (as well as IL-2). We note that S. hasekei n. sp. is not a member of the IIIP-1 clade, which includes numerous European members in the S. vernum group (Adler et al. 2016, Seitz & Adler 2017).
Distribution and bionomics. Simulium hasekei n. sp. is known from only one small alkaline ponor brook (discharge <2 l/sec, conductivity 220–255 µS/cm) surrounded by peatland with mountain pines and meadows in the Northern Limestone Alps of Austria (“Eastern Dachstein Plateau”, Fig. 18 View FIGURE 18 ). Every year, the water seeps away in a short time during June or July, making it difficult to collect larvae of adequate size for chromosomal analysis and mature pupae. This was the reason why the search for immature stages in 2017 and 2018 was unsuccessful. In addition to our new species, the brook harboured Simulium aureum (Fries) s. s., Simulium beltukovae (Rubtsov) , and Simulium vernum Macquart s. s. ( Seitz 2019, Tab. 10 “MIBOMOPO”). The new species is univoltine, as the area tends to form pronounced cold air lakes due to the geological boundary conditions, and this condition contributes to delaying the temperature-controlled development of the larvae over the year ( Prenner 2011). Although the feed- ing habits of the females are unknown, the fully toothed mouthparts and basal, thumblike lobe on each tarsal claw suggest ornithophily. With the discovery of S. hasekei n. sp., 52 species of Simuliidae are now known from Austria (cf. Seitz 2019).
Given that the nearest known relatives are found primarily in northern environments, S. hasekei n. sp. might represent a relict species pushed south into Central Europe during the last glacial period. Or, it might be descended from northern faunal elements that moved into the mountains of Central Europe in the postglacial period ( Aspöck 2008).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |