Prognathorhynchus eurytuba Ax & Armonies, 1987
|
publication ID |
https://doi.org/10.5281/zenodo.200746 |
|
DOI |
https://doi.org/10.5281/zenodo.6189695 |
|
persistent identifier |
https://treatment.plazi.org/id/F24C880C-1F45-9D2E-6093-A43EFD07FE27 |
|
treatment provided by |
Plazi |
|
scientific name |
Prognathorhynchus eurytuba Ax & Armonies, 1987 |
| status |
|
Prognathorhynchus eurytuba Ax & Armonies, 1987 View in CoL
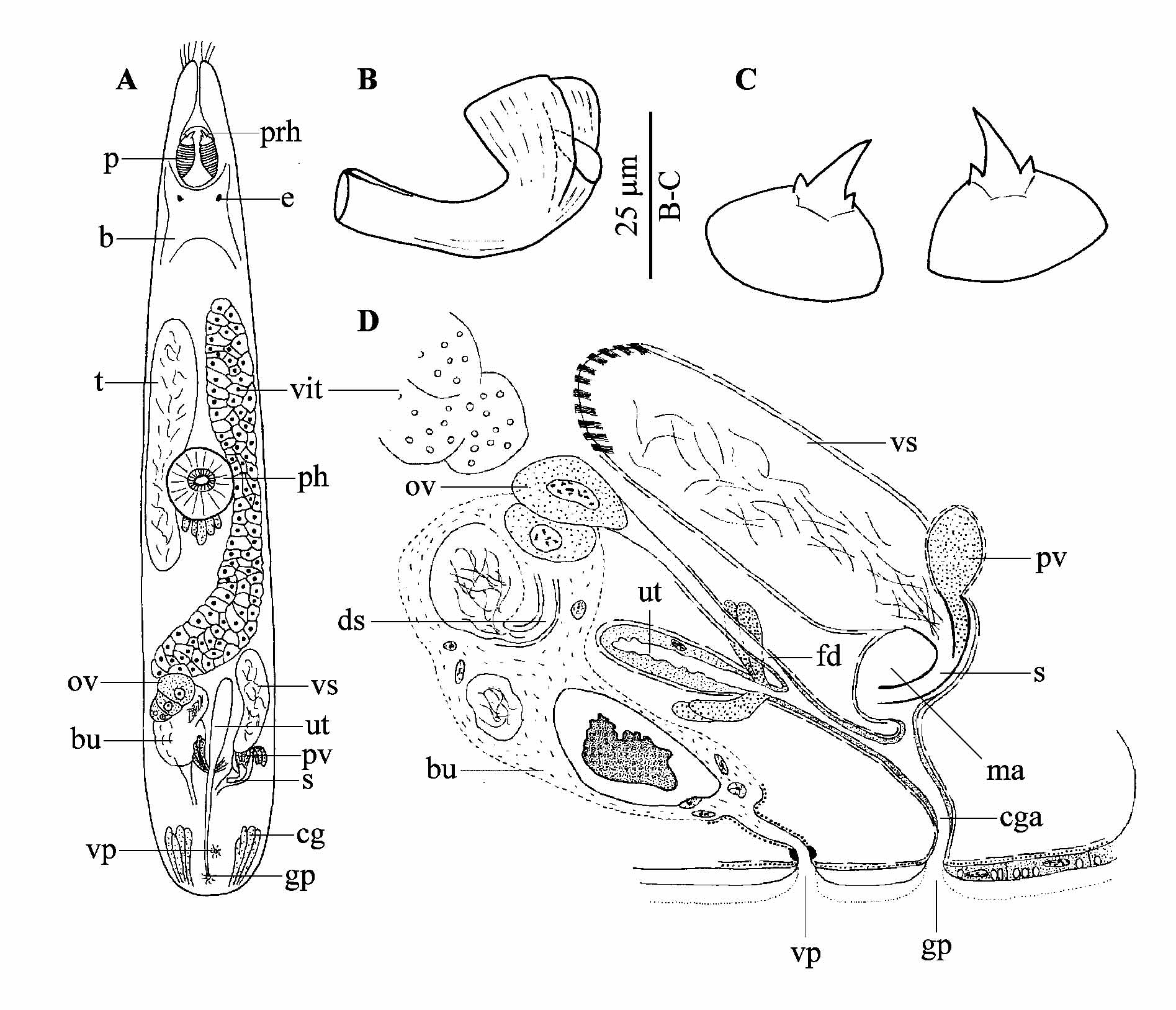
( Fig. 1 View FIGURE 1 )
New locality. Santa Cruz Island, Bahia Academy, stations IX5 e and IX 5 f.
Known distribution. North West Atlantic: several localities in New Brunswick, Canada and in South Carolina, USA (see Ax & Armonies 1987; Ax 1997).
Material. Four individuals studied alive, five sagittally-sectioned specimens ( ZMUG 23317-23321).
Remarks. The Galapagos specimens can easily be identified as P. e u r y t u b a, based on the overall organisation of the body, the morphology of the proboscis hooks and that of the stylet. As the original description by Ax and Armonies (1987) and a later account by Ax (1997) were based only on observations on live animals, very little is known of the detailed morphology, and a reconstruction of the genital system is lacking. The new material from the Galapagos allows a detailed and more complete description, although certain features are still hard to discern, especially concerning details of the female system, which appears to be difficult to study in members of the taxon Prognathorhynchus Meixner, 1929 (see Karling 1947; Brunet 1973; Ax & Armonies 1987).
Live animals about 0.7−0.8 mm long. The cellular epidermis (± 5 µm thick) is ciliated over the entire body (cilia ± 5 µm long). A few sensory bristles (± 20 µm long) are present around the proboscis opening. The proboscis hooks ( Fig. 1 View FIGURE 1 C) are as described by Ax and Armonies (1987), only slightly larger. The diameter of the basal plate is ± 25 µm ( Ax & Armonies 1987: 17 µm). The stylet’s ( Fig. 1 View FIGURE 1 B) shape is as illustrated by Ax and Armonies (1987). The stylet length in the Galapagos specimens varies from 42−48 µm ( x = 45 µm; n = 3), which is longer than in the Canadian ( Ax & Armonies 1987: 32 µm) and the specimens from South Carolina ( Ax 1997: 35 µm).
The common genital pore ( Fig. 1 View FIGURE 1 A, D: gp) is situated ventrally, very close to the caudal end and opens into a tubiform common genital atrium, which is lined with a low, anucleated epithelium and is surrounded by a longitudinal muscle layer. Basophilic caudal glands are situated on both sides of the genital atrium. The genital atrium receives the male system from the dorsal side, and both the female system and the uterus from the rostral side. The elongated seminal vesicle is surrounded by strong longitudinal muscles with an oblique orientation in the proximal part. Sperm is discharged through the funnel-shaped opening of the stylet (see also Ax & Armonies 1987 and Ax 1997), whereas the globular prostate vesicle, which contains a coarse-grained basophilic secretion and which is surrounded by circular muscles, discharges through the second, smaller opening of the stylet. The stylet lies in the oviform male atrium, which is surrounded by longitudinal muscles and lined with a very low, anucleated epithelium. From the single ovary, the female duct, which is lined with a very low, anucleated epithelium and only distally surrounded by longitudinal muscles, runs ventro-caudally towards the common genital atrium. A very large and vaguely defined bursa contains numerous large nuclei and sperm in different stages of degeneration as well as live sperm, organized in separate compartments. From one of these compartments a curved spermatic duct ( Fig. 1 View FIGURE 1 D: ds), surrounded by longitudinal muscles, runs towards the ovary, although the connection proper is hard to discern. A vaginal pore ( Fig. 1 View FIGURE 1 A, D: vp), situated rostrally from the common genital pore, is clearly visible on all sectioned animals, but could only be observed in one out of four live specimens. It is surrounded by a strong sphincter and continues into a vaginal duct, which is surrounded by circular muscles and which leads to the bursa.
In the diagnosis for the taxon Gnathorhynchidae by Karling (1947), the presence of only a single genital pore is mentioned. Also Ax and Armonies (1987) and Ax (1997) do not mention a vaginal pore for P. eurytuba . However, a second opening of the genital system (i.e. a vaginal pore as in P. e u r y t u b a) is also observed by Hochberg (2004) in P. busheki Ax, 1997 , using confocal laser scanning microscopy. Apart from P. busheki and P. e u r y t u b a at least two other species of Prognathorhynchus have a female bursa functioning as a seminal receptacle: P. parvulus Brunet, 1973 and P. dividibulbosus Ax & Armonies, 1990 . As the vaginal pore is often very difficult to see in live specimens, and many of the species of Prognathorhynchus were described without sectioned material, it is possible that a vaginal pore is present in more (even all?) species of this taxon, especially in those in which the presence of a female bursa has been recorded. It is, therefore, apparent that the taxa Prognathorhynchus and Gnathorhynchidae are highly in need of taxonomic revision.
It should be checked on animals from the Atlantic populations of P. e u r y t u b a whether they indeed are identical in all details to the population from Galapagos, and hence indeed belong to the same species. The disparate distribution, indeed, could indicate a geographical isolation of both populations. At the moment, however, we cannot but consider the Galapagos population as belonging to the same species, as at this moment no fixed morphological differences between the two populations can be found.
| ZMUG |
Zoologisches Museum der Universitat Gottingen |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Kalyptorhynchia |
|
Family |
|
|
Genus |