Boophis luciae, Glaw & Köhler & Riva & Vieites & Vences, 2010
|
publication ID |
https://doi.org/ 10.11646/zootaxa.2383.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/F566C51E-FFA0-FFE1-E883-FA00919711A0 |
|
treatment provided by |
Felipe |
|
scientific name |
Boophis luciae |
| status |
sp. nov. |
Boophis luciae View in CoL sp. nov.
( Fig. 26 View FIGURE 26 , Appendix 9)
Remark. This species has been referred to as Boophis sp. aff. sibilans by Glaw & Vences (2007:172–173) and as Boophis sp. 17 in Vieites et al. (2009).
Holotype. ZSM 327 View Materials /2000, adult male, from Vohidrazana , 18°57'57'' S, 48°30'37'' E, 731 m a.s.l., eastern Madagascar, collected on 10 April 2000 by F. Glaw. GoogleMaps
Paratypes. UADBA ( ZCMV 688 ), sex unknown, from near Ambatolahy , 21°14.632' S, 47°25.573' E, 915 m a.s.l., collected on 10 February 2004 by D GoogleMaps . R. Vieites and C. Woodhead ; ZFMK 60126–60129 About ZFMK and 60834, adult males, from Ranomena near Andasibe, collected on 2 April 1995 by F. Glaw and D. Vallan ; ZFMK 64141 About ZFMK , male, from the Ranomafana region , collected on 3–4 March 1996 by F. Glaw, D. Rakotomalala and F. Ranaivojaona ; ZFMK 60041 About ZFMK , adult male, from Andasibe , collected on 1 February 1995 by F. Glaw ; ZFMK 62307 About ZFMK , 62908 About ZFMK , adult males, from Ranomafana , collected on 2 March 1996 by F. Glaw, D. Rakotomalala and F. Ranaivojaona ; ZMA 20305 ( ZCMV 595 ), adult female , ZMA 20306–20307 View Materials ( ZCMV 687 , 689 ), adult males, from near Ambatolahy , 21°14.632' S, 47°25.573' E, 915 m a.s.l., collected on 9 February 2004 by D GoogleMaps . R. Vieites and C. Woodhead ; ZSM 63 View Materials /2004, adult male, from Andohahela , between Isaka and Eminiminy, Camp 1, 24°45'31'' S, 46°51'15'' E, 247 m a.s.l., collected on 29–31 January 2004 by F. Glaw, M. Puente GoogleMaps , R. Randrianiaina and M. Thomas ; ZSM 328–329 View Materials /2000, adult males, same data as holotype GoogleMaps ; ZSM 243 View Materials /2006 ( ZCMV 3033 ), adult male , ZSM 244 View Materials /2006 ( ZCMV 3057 ), adult female, from near Ambatolahy , 21°14.632' S, 47°25.573' E, 915 m a.s.l., collected on 26 February 2006 by P. Bora, Y. Chiari, E. Rajeriarison GoogleMaps , T. Razafindrabe and M. Vences .
Etymology. Ignacio De la Riva dedicates this species to his daughter Lucia, as compensation for the time he spends doing field work abroad, something she rather dislikes.
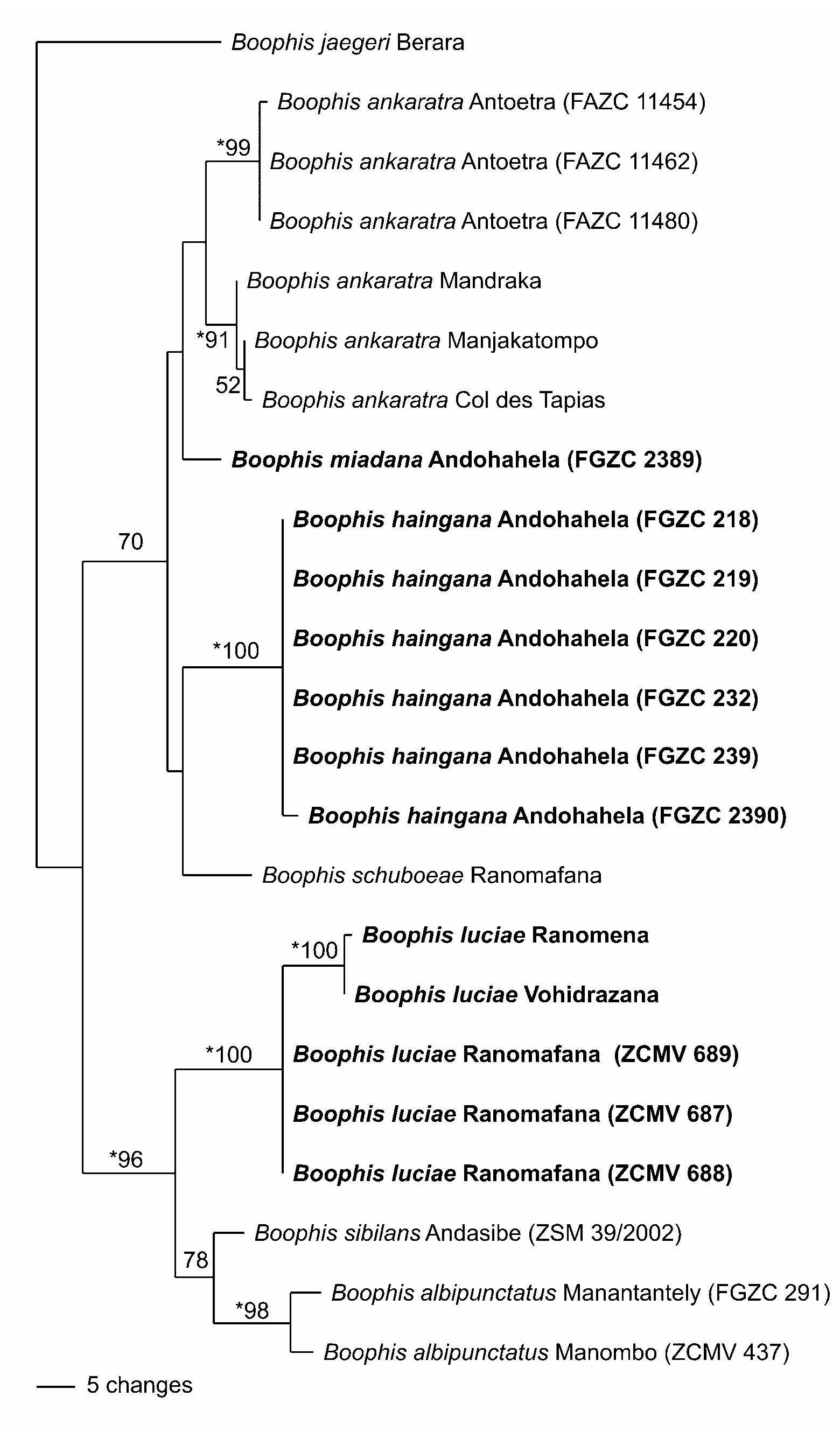
Diagnosis. Assigned to the genus Boophis based on the presence of an intercalary element between ultimate and penultimate phalanges of fingers and toes (verified by external examination), absence of femoral glands in males, absence of gular glands in males, presence of axillary amplexus, enlarged terminal discs of fingers and toes, lateral metatarsalia separated by webbing, absence of outer metatarsal tubercle, molecular phylogenetic relationships (see Vieites et al. 2009 for a complete molecular analysis of Boophis ), and overall similarity to other Boophis species. Assigned to the Boophis albipunctatus group based on the following combination of characters: small size (male SVL 24–31 mm); absence of tubercles or flaps on heel and elbow; presence of webbing between fingers; indistinct canthus rostralis; dorsal colouration translucent green with many small white dots in life and yellow-whitish in preservative; absence of red ventral colour; ventral skin in life non-transparent; single subgular vocal sac; presence of vomerine teeth; molecular phylogenetic relationships; and its high morphological similarity to B. albipunctatus and B. sibilans . The new species differs from all other species in the Boophis albipunctatus group by its strong genetic differentiation (see below). Along a stream near Andasibe, B. luciae occurs in syntopy with B. albipunctatus and B. sibilans . Within the B. albipunctatus group, the new species belongs to a clade of morphologically very similar species ( B. albipunctatus , B. luciae , B. sibilans ) characterized by (1) presence of well-defined brown markings on the iris, and (2) presence of a dense dorsal spotting with small and sharply defined white spots. Within this complex of species, B. luciae is characterized by its usually smaller body size (male SVL of B. luciae 24–31 mm, mostly below 30 mm, vs. 28–34 mm in the other two species). It is further distinguished by its advertisement call: from B. albipunctatus , by a longer duration and different structure of notes (trill notes vs. short clicks; note duration 18– 101, average 61 ms, vs. 19–43 ms), and from B. sibilans by the pulsed structure of its trill notes (vs. tonal unpulsed whistles; see Comparative call data below).
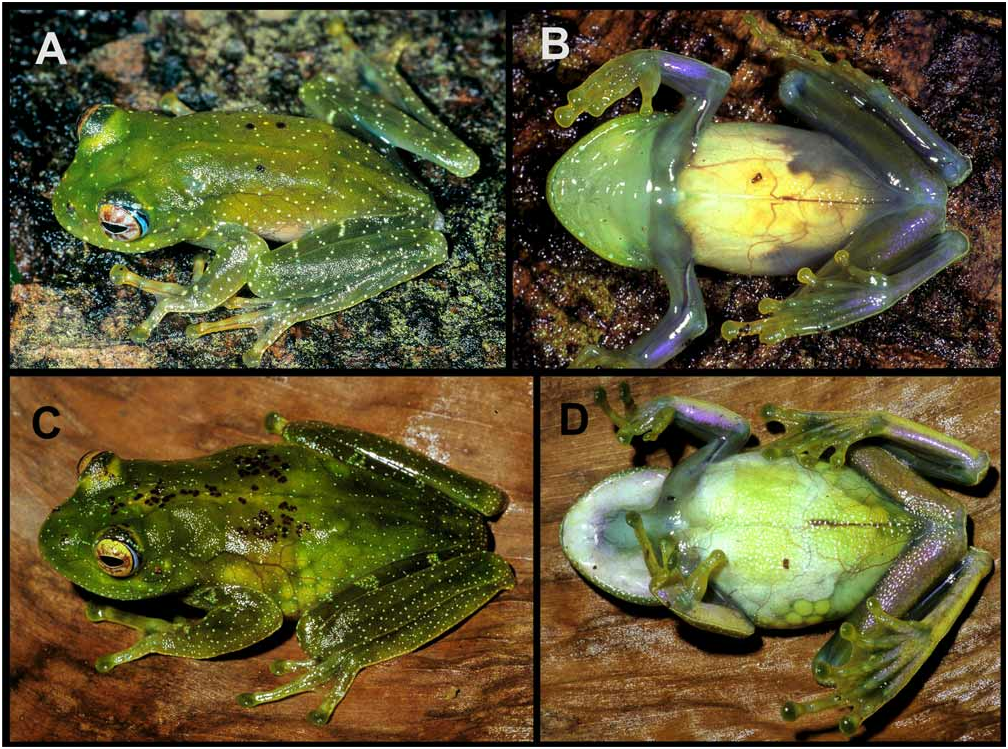
Description of the holotype. Adult male, SVL 28.5 mm. Body moderately slender; head as longer as wide, slightly wider than body; snout rounded in dorsal and lateral view, nostrils directed laterally, slightly nearer to tip of snout than to eye; canthus rostralis rounded in cross section, straight in dorsal view, loreal region slightly concave; tympanum distinct, round, TD 45% of ED; supratympanic fold indistinct; vomerine odontophores distinct, well separated in two elongated patches, positioned posteromedial to choanae; choanae medium-sized, rounded. Tongue posteriorly bifid, free behind. Arms slender, subarticular tubercles single, round; metacarpal tubercles not recognizable; fingers moderately webbed and with lateral dermal fringes; webbing formula 1(1), 2i(1.25), 2e(0.75), 3i(1.25), 3e(1.5), 4(1.25); relative length of fingers 1<2<4<3 (finger 2 distinctly shorter than finger 4); finger discs enlarged. Hindlimbs slender; tibiotarsal articulation reaching beyond the tip of snout when hindlimb is adpressed along body; lateral metatarsalia separated by webbing; inner metatarsal tubercle small, distinct, elongated; no outer metatarsal tubercle; toes broadly webbed; webbing formula 1(0), 2i(0.5), 2e(0.25), 3i(0.25), 3e(0), 4i(1), 4e(1), 5(0.25); relative length of toes 1<2<5=3<4; toe discs enlarged. Skin smooth on dorsal surfaces, granular on throat, chest, belly and ventral surfaces of thighs. The entire left forelimb was removed for tissue sample.
Measurements (in mm): SVL 28.5, HW 10.6, HL 10.6, ED 3.3, END 2.3, NSD 2.2, NND 3.0, TD 1.5, TL 45.8, HAL 9.8, FOL 12.6, FOTL 20.8.
After seven years in preservative, ground colour of upper and lower surfaces of head, dorsum, and limbs creamy yellow, with small dark spots or flecks on flanks, lips, tympanic region, and limbs; small brown flecks on central dorsum and around nostrils. Posterior part of eye purplish. There are no data on colour in life of the holotype.
Variation. Morphometric variation is given in Appendix 7. Females are larger, at average having approximately 154 % of male SVL. Overall, the paratypes are similar to the holotype. In life, dorsal surfaces of head, body and limbs translucent green with scattered small, cream dots; there are few scattered brown dots on head and dorsum, often especially concentrated in a group at each side of mid-dorsum. Upper surface of fingers, toes and terminal discs the same colour as dorsum. Lower flanks transparent. Ventral skin transparent, ventral peritoneum white. Ventral skin of limbs translucent with a bluish tone. Iris golden (orange near the border), with purple markings, more densely arranged around the pupil; eye periphery black posteriorly, then blue, and again black ( Fig. 26 View FIGURE 26 ). In calling males we observed a highly extensible single subgular vocal sac.
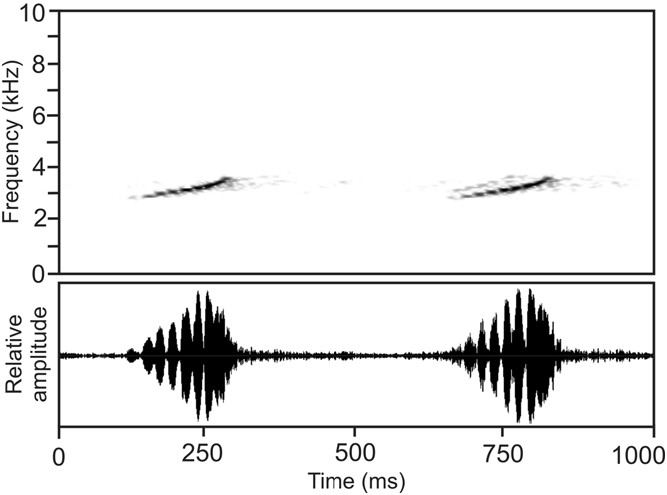
Natural history. Similar to Boophis albipunctatus and B. sibilans , calling males of B. luciae are often heard at night from high perches up to 4 m in the vegetation along streams in primary and degraded rainforest. In most cases, it is very difficult to observe the calling males, although along some streams this species occurs in rather high densities and non-calling specimens and pairs in axillary amplexus can be observed later at night on lower perches. Especially in the early evening, single individuals may emit only one of the two possible call types (see below).
Vocalization. Generally, calls of Boophis luciae ( Fig. 27 View FIGURE 27 ) may contain three different call types. Type 1 consists of a long series of trill notes (reminding whistles if heard in the field from a distance), emitted in regular intervals. Each trill note contains 5–10 distinctly separated pulses, whereby calls from the Ranomena population contain 5 pulses only. Call type 2 is a short series (maximum 1710 ms duration) of 9–13 whistles, with an increase in note duration towards the end of the series. Call type 3 consists of a short series of 3–6 click notes separated by long intervals, mostly followed by 1–2 whistles, or sometimes followed even by a long series of whistling notes as described for call type 2. Trills and whistles in calls of B. luciae show distinct frequency modulation, with frequency constantly increasing towards the end of the note. Numerical parameters for the different call types of the Ranomena (near Andasibe) population are as follows: Call type 1: note duration, 146–201 ms (176 ± 13; n = 58); inter-note intervals, 288–497 ms (376 ± 41; n = 55); note repetition rate, 1.7–2.0 notes/second. Call type 2: note duration, 18–101 ms (61 ± 18; n = 76); inter-note intervals, 39– 106 ms (73 ± 16; n = 68); note repetition rate, 7.5–9.8 notes/second. Call type 3: note duration, 15–39 ms (27 ± 7; n = 18); inter-note intervals, 395–527 ms (459 ± 36; n = 13). Dominant frequency range 3000–4500 Hz, maximum call energy at 3600–4300 Hz. Numerical parameters for the different call types of the Vohiparara (Ranomafana) population are as follows: Call type 1: note duration, 152–213 ms (179 ± 12; n = 55); inter-note intervals, 250–529 ms (398 ± 43; n = 48); note repetition rate, 1.6–2.0 notes/second. Call type 2: note duration, 23–81 ms (61 ± 10; n = 48); inter-note intervals, 62–103 ms (84 ± 11; n = 43); note repetition rate, 6.6– 7.6 notes/second. Call type 3: note duration, 26–41 ms (31 ± 7; n = 4); inter-note intervals, 467–599 ms (546 ± 70; n = 3). Dominant frequency range 2950–3900 Hz, maximum call energy at 3150–3800 Hz (see Vences et al. 2006, CD 1, track 27).
Comparative call data. In comparison to sympatric Boophis sibilans from Andasibe, the call type 1 of B. luciae contains distinctly pulsed trill notes (vs. unpulsed whistles) which are usually emitted in much faster succession (mean inter-note interval 656 vs. 375 ms). Moreover, frequency modulation within whistles is far less pronounced in B. sibilans . Click notes are so far unknown in the latter species. For better comparison and in addition to the data provided by Glaw & Thiesmeier (1993), we here provide a more detailed analysis of call type 1 and 2 of B. sibilans from Andasibe (type locality): Call type 1: note duration, 151–186 ms (164 ± 9; n = 34); inter-note intervals, 502–931 ms (656 ± 132; n = 33); note repetition rate, 0.9–1.6 notes/second. Call type 2: note duration, 32–150 ms (117 ± 30; n = 14); inter-note intervals, 113–239 ms (137 ± 37; n = 13); note repetition rate, 3.8–5.0 notes/second; maximum duration of call series, 3436 ms (14 notes, 4.1 notes/second). Dominant frequency range 2600–3450 Hz, maximum call energy at 2700–3300 Hz. Harmonic frequency bands are present at 5600–6100 Hz and 8450–9150 Hz ( Vences et al. 2006, CD 1, track 26).
Molecular relationships. The phylogenetic tree of species assigned to the Boophis albipunctatus group ( Fig. 21 View FIGURE 21 ) provides evidence for two subclades that correspond well with morphological characters. The first subclade (supported by a bootstrap value of 70% but not by the Bayesian analysis) comprises B. ankaratra , B. schuboeae , and allied species (two of which are described as new herein, B. miadana and B. haingana ); all these species are green with an irregular pattern of light mottling or with few to many (usually) ill-defined light dots on a green ground colour. The second subclade (BS 96%, BPP significant) contains three species characterized by a pattern of many very small but well-defined white dots on a green ground colour. Of these three species, B. sibilans and B. albipunctatus are grouped together (BP 78%, BPP not significant), whereas B. luciae is most basal. Genetic divergences of B. luciae are 4.8–6.2 to B. sibilans and 6.4–7.6% to B. albipunctatus . Boophis sibilans and B. albipunctatus differ by 3.5–3.8%. The two specimens of B. albipunctatus differ by 1.4% whereas the specimens of B. luciae from the Andasibe region (Ranomena and Vohidrazana) differ by 1.9–2.4% from those of the Ranomafana region.
Comparative material. So far, B. albipunctatus is known from few localities only ( Andohahela, Manantantely, and Nahampoana in the South East; and Andasibe in the Northern Central East, where it occurs sympatrically with B. sibilans and B. luciae ). We here provide measurements of one further specimen from Ambohitsara (ZSM 2290/2007) in the Southern Central East which is the first record of the species from this region. It was collected from a chorus of various individuals emitting the typical calls as described by Glaw & Thiesmeier (1993). Furthermore, one DNA sequence (see Fig. 21 View FIGURE 21 ) of a specimen collected in Manombo Special Reserve, a coastal locality in South Eastern Madagascar, provides evidence for the occurrence of the species at this locality.
Distribution. Boophis luciae is known from Ambatolahy and other sites in and near Ranomafana National Park, from Andohahela National Park at lower elevations based on call recordings, and from An'Ala, Andasibe, Vohidrazana and Ranomena (near Andasibe) (Appendix 10).
Available names. Several names in the Boophis luteus group are potentially available as earlier names for the following four new species described in this paper: Boophis sandrae , B. miadana , B. haingana , and B. luciae . All species of the Boophis luteus group quickly loose the greenish colouration after preservation and fade to yellowish-white. For this reason and due to their morphological similarities it is notoriously difficult to identify preserved voucher specimens for which additional data are unknown. A lectotype (BMNH 1947.2.9.7) of Rhacophorus luteus var. longicrus Parker, 1925 was designated by Glaw & Vences (1992): Adult male (with nuptial pads recognizable on the first finger), SVL 33.2 mm, eye diameter 4.4 mm, eye-nostril distance 2.4 mm, nostril-snout tip distance 3.1 mm, tympanum diameter 1.6 mm, flanks with remains of white marbling, iris periphery with remains of blue colour. This taxon is considered as a junior synonym of Boophis luteus since long. Its small SVL (adult male 33.2 mm vs. 31.9–37.0 mm in B. luteus ) and relatively small eyes (eye-diameter / SVL = 0.13 vs. 0.11–0.12 in B. luteus ) argue for this synonymy and against an attribution to Boophis sandrae (male SVL 35.5–48.2 mm, eye-diameter / SVL = 0.11–0.15) or B. elenae (SVL 40.4–46.0 mm, eye-diameter / SVL = 0.11–0.12). Rhacophorus anceps Mocquard, 1902 (type locality Fort Dauphin) and Rhacophorus isabellinus Boettger, 1913 (type locality Moramanga) are based on single, poorly preserved and probably juvenile specimens. The former taxon was repeatedly considered as a dubious name ( Guibé 1978, Blommers-Schlösser & Blanc 1991, Andreone 1993). An unambiguous attribution of both these taxa to any species of the B. luteus group appears impossible. We continue considering them as junior synonyms of Boophis luteus .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.