Atopopus tarsalis Eaton, 1881
|
publication ID |
https://doi.org/10.5281/zenodo.178454 |
|
DOI |
https://doi.org/10.5281/zenodo.6250964 |
|
persistent identifier |
https://treatment.plazi.org/id/F66CFB09-C36A-FFA2-8B8C-4C5C3032A7ED |
|
treatment provided by |
Plazi |
|
scientific name |
Atopopus tarsalis Eaton, 1881 |
| status |
|
Atopopus tarsalis Eaton, 1881 View in CoL
Nymph. Size: male, body length up to 12.3 mm, cerci length up to 14.8 mm; female, body length up to 14.9 mm, cerci length up to 15.5 mm.
Head: Broader than the pronotum, antennae short, at most reaching the margin of the cephalic capsule. Hind margin smoothly concave, lateral emargination behind the eyes strongly developed ( Fig. 1 View FIGURES 1 – 10 ). Fore margin above
Labrum expanded laterally, about 5 times broader than long, slightly bent backwards ( Fig. 2 View FIGURES 1 – 10 ). Anterior margin with a V-shaped incision densely covered with long and thin setae. Ventral surface with submarginal row of stout setae arranged in V-shape.
Mandibles slender and elongated, outer margin densely and regularly covered with long setae. Left mandible with well developed inner incisor; outer incisor serrated on its entire inner face; tuft of 5–7 somewhat plumose setae below the inner incisor; 3–5 hair-like setae below the mola ( Fig. 3 View FIGURES 1 – 10 ). Right mandible with shorter inner incisor, ending with two setae-like teeth; outer incisor serrate on its inner face, except the apical fourth; tuft of 7–9 plumose setae below the inner incisor; tuft of numerous, short setae above the mola, and a row of 7–9 hair-like setae below it; mola ending posteriorly with a hook ( Fig. 4 View FIGURES 1 – 10 ).
Ventral surface of the galea-lacinia covered with scattered simple setae; proximal dentiseta forked near apex; distal dentiseta composed of a bunch of forked and simple setae (fig. 5); crown of the galea-lacinia covered with 15–19 comb-shape setae, the median ones composed of ca 10–12 teeth; first segment of the maxillary palp with numerous long setae on its outer margin, and with scattered and thinner setae on its inner one.
Lingua of hypopharynx thick and with a tuft of setae apically; superlingua well developed, outer margin folded one or two times, apex pointed and strongly bent backwards ( Fig. 6 View FIGURES 1 – 10 ).
Labium with glossae widely separated by a large and angular emargination; inner face of glossae covered with long setae; paraglossae extended laterally, with rounded apex and slightly turned backwards.
Thorax: Lateral margin of the pronotum rounded, without postero-lateral expansions; posterior margin slightly concave.
Coxae increasing in size from the fore to the hind coxae, acuminated posteriorly.
Legs unusually short and stout; ratio length/width of the fore, middle and hind femora ca 2.0, and ratio femur/tibia lengths ca 1.3 ( Fig. 7 View FIGURES 1 – 10 ). Dorsal margin of femora covered with long and stout setae, ventral margin with scattered small spine-like setae, increasing in number from the fore- to the hind femora; upper face covered with numerous small spine-like setae, rounded at the apex, slightly longer than wide. Dorsal margin of tibiae covered with long and thin setae, ventral margin with few spine-like setae. Tarsi with dorsal margin covered with long and thin setae, except the fore tarsi which bear only minute thin setae. Presence of a well developed spine-like seta on the ventro-distal part of the hind tarsi. Tarsal claws without apical denticle.
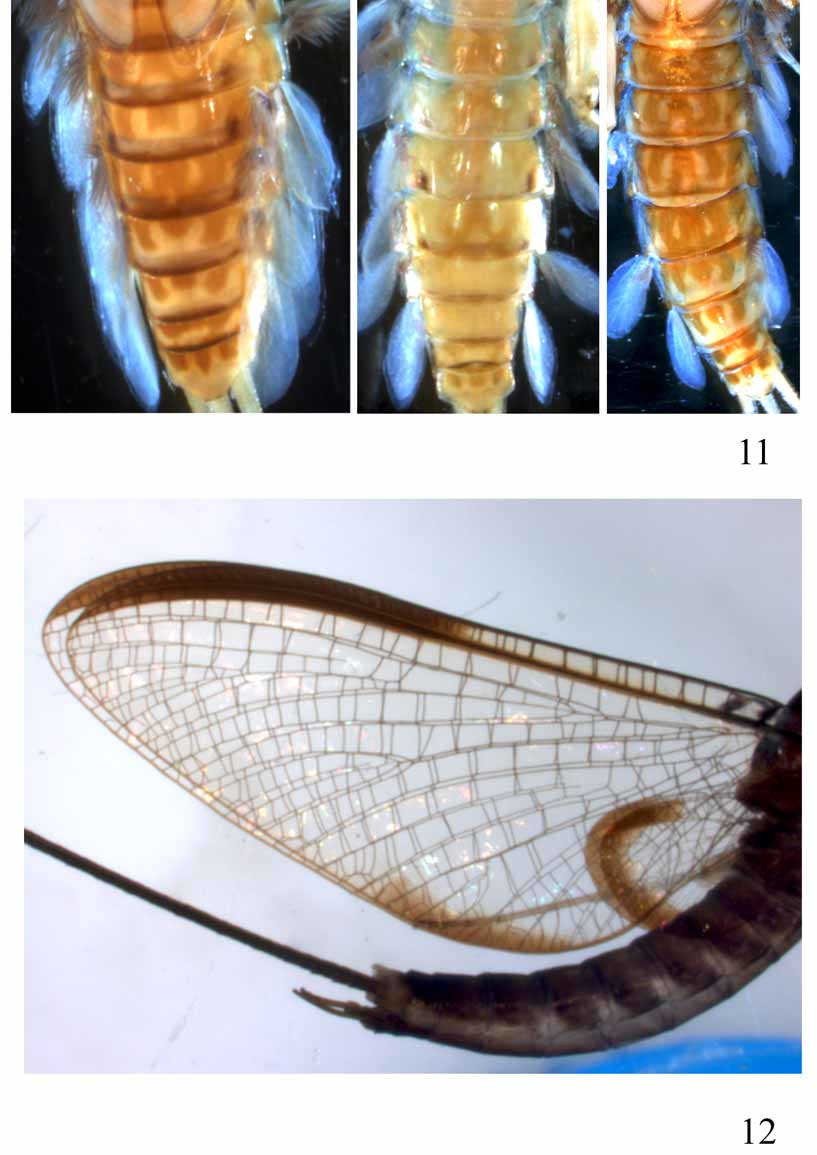
Abdomen: Colour pattern very characteristic and as in Fig. 11 View FIGURES 11 – 12 . Abdominal sternite I with lateral sclerites quadratic to pentagonal, and reaching almost the posterior margin. Postero-lateral extensions increasing in size from sternite II to VIII ( Fig. 8 View FIGURES 1 – 10 ).
Gills present on segments I–VII. Gill I attached dorsally and composed of a well developed tuft of fibrillae and a minute, almost invisible lamella ( Fig. 9 View FIGURES 1 – 10 ). Gills II–VI alike, attached laterally and composed of a lamella with well marked tracheation, and a tuft of purple fibrillae ( Fig. 10 View FIGURES 1 – 10 ). Gill VII with a well developed lamella but without fibrillae. All lamellae with some kind of strengthening subparallel to the outer margin.
Posterior margin of the sternite IX of female nymphs slightly concave.
Cerci and well developed terminal filament with whorls of spine-like setae, without hair-like setae.
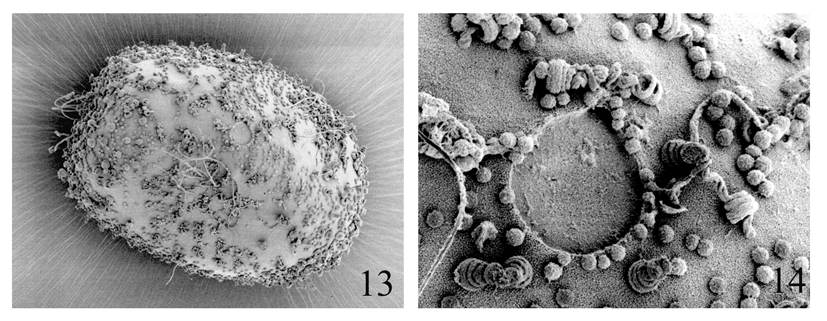
Egg. Shape ovoid, ca 110 μm x 75 μm ( Fig. 13 View FIGURES 13 – 14 ). Chorionic surface covered with small granulations (ca 1 μm in diameter), as well as with medium size knob-terminated coiled threads (KCT’s) approximately 2–3 μm in diameter. Two polar caps with numerous KCT’s of the same size as those on the other part of the chorion. Four micropyles situated in the equatorial area; margin of the micropyle with irregularly placed granulations ( Fig. 14 View FIGURES 13 – 14 ).
Material examined. All the material listed below comes from the Island of Borneo and is deposited in the Museum of Zoology, Lausanne, Switzerland and in the Museum of Zoology, Bogor ( LIPI), Indonesia.
INDONESIA, East Kalimantan: Malinau Basin
Rian River tributaries
Belakau, Langap Sud (1997-petak 6), 116°30'26''E, 3°,04'04''N, 13.06.2000 (B0110), Derleth, P., 10 nymphs; same locale but 0 5.07.2000 (B0111), Derleth, P., 7 nymphs; same locale but 20.04.2001, (B0113), Derleth, P. & Sartori, M., 10 nymphs; same locale but 0 7.07.2000, (0121), Derleth, P., 10 nymphs; same locale but 18.04.2001 (B0123), Derleth, P. & Sartori, M., 10 nymphs
Ngayo, Langap Sud (1995), 116°31'11''E, 3°04'41''N, 14.06.2000 (B0410). Derleth, P., 5 nymphs; same locale but 0 8.07.2000, (B0411), Derleth, P., 12 nymphs;
Ngayo, Langap Sud (1995), 116°31'15''E, 3°04'41''N, 12.07.2000. (B0421), Derleth, P., 12 nymphs
Unnamed tributary, Langap Sud (1999-petak 24), 116°31'05''E, 3°01'40''N, 11.07.2000, (B1211), Derleth, P., 19 nymphs.
Seturan River tributaries
Temalat (Sungai Guang), Seturan (1999-petak 39-40), 116°32'24''E, 3°00'10''N, 0 1.07.2000, (B0211), Derleth, P., 1 nymph; same locale but 27.03.2001, (B0213), Derleth, P., 2 nymphs;
Seturan River main stream, Bulungan camp, 116°30'36''E, 3°00'20''N, 13.07.2000, (B0431), Derleth, P., 12 nymphs
Tamalang, Seturan (2001-petak 57), 116°30'29''E, 2°59'22''N, 10.04.2001, (B0513), Derleth, P., 1 nymph;
Bengahau, Seturan (2001-petak 57), 116°30'46''E, 2°59'22''N, 0 8.08.2000, (B0531), Derleth, P., 2 nymphs; same locale but 11.04.2001, (B0533), Derleth, P. & Feldmeyer, B., 7 nymphs
Wok (Sungai Guang), Seturan (2000-petak 45), 116°33'29''E, 2°59'09''N, 16.06.2000, (B0611), Derleth, P. & Gattolliat, J.-L., 1 nymph;
Wok (Sungai Guang), Seturan (2000-petak 45), 116°33'30''E, 2°59'11''N, 29.06.2000, (B0631), Derleth, P., 11 nymphs
Wok (Sungai Guang), Seturan (2000-petak 44-45), 116°33'11''E, 2°59'12''N, 17.06.2000, (B0711), Derleth, P. & Gattolliat, J.-L., 8 nymphs; same locale but 0 5.04.2001, (B0713), Derleth, P. & Feldmeyer, B., 29 nymphs;
Temalat (Sungai Guang), Seturan (2000-petak 43), 116°33'29''E, 2°59'29''N, 18.06.2000, (B0811), Derleth, P. & Gattolliat, J.-L., 10 nymphs; same locale but 16.08.2000, (B0812), Derleth, P., 11 nymphs; same locale but 16.04.2001, (B0813), Derleth, P., 29 nymphs; same locale but 21.06.2000, (B0821), Derleth, P. & Gattolliat, J.-L., 6 nymphs; same locale but 0 4.04.2001, (B0823), Derleth, P., 3 nymphs; same locale but 16.04.2001, (B0833), Derleth, P. & Sartori, M., 6 nymphs.
Unnamed tributary, Seturan (unexploited), 116°33'29''E, 2°58'54''N, 24.04.2001, (B1413), Derleth, P., Sartori, M. & Feldmeyer, B., 1 male imago, 8 nymphs;
Unnamed tributary, Seturan (unexploited), 116°33'30''E, 2°58'58''N, 26.04.2001, (B1423), Derleth, P. & Sartori, M., 2 nymphs.
MALAYSIA — Sabah: Penampang River, Crocker Range National Park, 15.08.2003, (B2001), M. Whiting's lab., 5 nymphs.
Affinities. The nymphs of A. tarsalis are more similar to those of A. edmundsi than to those of A. tibialis or A. meyi based on the similarities in the number of comb-like setae on the galea-lacinia, the shape of the coxae and the shape of the 9th sternite of mature females. Based on the figures present by Wang and McCafferty (1995), there are distinct differences between A. edmundsi and A. tarsalis in the shape of the hypopharynx, the shape of the pronotum, the setation of the mandibles, and the relative size of the femora. In order to confirm these differences, we examined the type series of A. edmundsi and found that all of the differences indicated by the Wang and McCafferty (1995) figures are the result of inaccurate illustrations or poor specimens. Their figure of the hypopharynx does accurately represent the specimen from which it was drawn, but the specimen was killed in the process of moulting and the hypopharynx is distorted; we have also observed this distortion in mature larvae within large series of specimens that all have the typical posteriorly directed, sharply pointed lobes of the superlingua, so is it not likely a specific, but rather an artifact of moulting. In all examined characters, the paratype nymphs of A. edmundsi appear to be identical to those of A. tarsalis . Neither the association of the nymphs and adults of either A. edmundsi nor A. tarsalis was confirmed through rearing. We believe, however, that the nymph of A. tarsalis described herein is correctly associated with the adult as it was collected at essentially the same location as an adult male, whereas the nymphs and holotype male of A. edmundsi were collected from different locations. It is likely that the association made by Wang and McCafferty (1995) is incorrect and they inadvertently described the nymph of A. tarsalis .
The adult male of A. edmundsi appears to be a good species, and differs from males of A. tarsalis in having the first segment of the hind tarsus shorter than the hind tibia. Additionally, the fore wing is approximately 3.5 times longer than wide, whereas in A. tarsalis the fore wing is less than 3 times longer than wide ( Fig. 12 View FIGURES 11 – 12 ). The narrow hind wing described and illustrated by Wang and McCafferty (1995) is inaccurately drawn; the wing from which the drawing was made is folded, making it appear narrower than it actually is. The other hind wing of the holotype is not folded and has the same shape as those of A. tarsalis . The female subimago described by Wang and McCafferty (1995) as A. edmundsi has fore wings that are 2.8 times longer than wide, so is unlikely to actually be A. edmundsi . It is probable that this specimen is A. tarsalis , but because the female of A. tarsalis is unknown, we cannot confirm this. Until A. edmundsi becomes better known, the species concept should be restricted to that of the holotype.
Distribution and ecology. Atopopus tarsalis is endemic to Borneo and is herein reported for the first time from Indonesia (East Kalimantan) although its presence was expected, based on its occurrence in Malaysia (Sabah).
Nymphs of this species have been found in small to medium size streams as well as in rivers in lowland dipterocarp forests. The watercourses ranged from 0.5–15 m wide, 2–80 cm deep, had current speeds between 0.2–1.4 m /s and water temperatures between 23.8–26.5°C. Despite this wide range of habitats, A. tarsalis has been shown to be a sensitive species, mainly present in pristine habitats (reference sites “group green” according to Derleth 2003).
A striking characteristic of this species is that 94% of all specimens were collected during qualitative prospecting whereas only 6% were collected with a Surber net. Detailed field observations show that A. tarsalis microhabitats are not located in small to medium size substrata where quantitative samples have been performed, but rather on and around large rocks and boulders (see below).
No adults of A. tarsalis were collected in light traps at either dusk or at dawn. The single male imago was caught resting under a leaf in the forest around noon. Nuptial flight probably occurs during the afternoon.
Behaviour. Nymphs of A. tarsalis have been mainly found scraping periphyton on large boulders. They generally are located a few centimetres below the water surface and, when disturbed, they move like crabs around the stone and are difficult to catch. What seems unique, to our knowledge, is that A. tarsalis nymphs, when not disturbed, are frequently found on rocks above the water surface. They can be found up to 20 centimetres above the water surface and in some occasions they were found at the top of the rock. As soon as they are approached too closely, they return to the water.
As far as we know, this behaviour has never been reported for any mayfly nymph. A South American mayfly, Cloeodes hydation McCafferty & Lugo-Ortiz, 1995 , a rock pool colonizer, has been reported to resist up to 9 hours out of the water ( Nolte et al. 1996). It seems to be an extreme adaptation to frequent drought in this peculiar habitat, and seems to be a passive behaviour. A. tarsalis behaviour is quite different since this species actively leaves the water and returns to it periodically, obviously to avoid dehydration. Our preliminary field observations, based on modest research facilities, indicate that A. tarsalis nymphs can spend up to 20 minutes out of the water, even in open streams and rivers with direct sunlight.
We have then to conclude that nymphs of A. tarsalis are able to retain a film of water around them to avoid complete dehydration. In that sense, the special shape of the head, with a thickened cephalic capsule, as well as the reinforcement of the gills may play a role in maintaining this water film and/or avoiding a total dehydration.
Reasons for this peculiar behaviour are not clear. Two hypotheses can be put forward. The first is that this behaviour allows A. tarsalis nymphs to avoid fish predation. The general behaviour of macroinvertebrates in the presence of carnivorous fishes is drifting (see e.g. Huhta et al. 1999; Huhta et al. 2000; McIntosh et al. 2002). This should be especially the case in rivers where fish composition is more diversified than in small streams (but see Melo & Froehlich 2004 for contrasting results). Climbing out of the water may be a less risky behaviour than drifting for avoiding predation for A. tarsalis .
Another hypothesis is that this behaviour may prevent A. tarsalis nymphs from being dislodged by the frequent and sudden spates that occur in the study area (pers. obs). When it is raining, the water level in streams and rivers increases rapidly, as do discharge and turbidity, inducing drift among macroinvertebrates and mayflies in peculiar (see e.g. Brittain & Eikeland 1988; Lancaster 1992).
But it is also possible that both hypotheses may be involved in this behaviour, as suggested by Dudgeon (1993). This will require complementary field studies in the future.
Finally it is worth mentioning that A. tarsalis is not the only mayfly that exhibits this behaviour. The baetid nymph Platybaetis probus Müller-Liebenau has been found generally together with A. tarsalis and is also frequently found above the water level. The morphological adaptation of this species will be discussed elsewhere.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |