Spirobranchus sinuspersicus, Pazoki & Rahimian & Struck & Katouzian & Kupriyanova, 2020
|
publication ID |
https://doi.org/10.11646/zootaxa.4748.3.1 |
|
publication LSID |
lsid:zoobank.org:pub:1A819C42-2B07-43DC-8670-9EA4805FA79F |
|
DOI |
https://doi.org/10.5281/zenodo.3705594 |
|
persistent identifier |
https://treatment.plazi.org/id/FA0F8798-5440-FFDB-499B-6389FE0C5A43 |
|
treatment provided by |
Plazi |
|
scientific name |
Spirobranchus sinuspersicus |
| status |
sp. nov. |
Spirobranchus sinuspersicus View in CoL sp. nov.
( Figs 2 View FIGURE 2 , 3 View FIGURE 3 A–D, 4, 5A–D, 6)
Synonymy.
Spirobranchus maldivensis View in CoL . —not Pixell, 1913 but sensu Wesenberg-Lund (1949): 358–359 [Jask, Gulf of Oman, Iran, diagno- sis].
Pomatoleios kraussii .— Mohammad (1971: 300) [ Kuwait, short description of tubes]; Mohammad (1975: 1–15) [ Kuwait, ecol- ogy]; Crisp (1977: 147–160) [ Kuwait, larval development]; Fiege (1992: 1–23) [ Saudi Arabia, “even found on heavily oiled rocks/beaches”]; Wehe & Fiege (2002: 129) [name only, checklist of Persian Gulf polychaetes], Al-Yamani et al. (2012: 75) [ Kuwait, name and photos of tubes]; Joydas et al. (2012: 330) [PG, Saudi Arabia, name only]; Safari et al. (2014a, b) [ Bandar Abbas, Strait of Hormuz, Iran, description].
Spirobranchus kraussii View in CoL .— Pillai (2009: 168, Fig. 49E–G) [ Abu Dhabi, United Arab Emirates, short description]; Baghernejhad (2012: 70) [ Bushehr, PG, Iran, name and abundance], Baghernejhad et al. (2015: 71, 73) [ Bushehr, PG, Iran, population dynamics]; Lavajoo & Amrollahi-Biuki (2015: 49–57) [ Bandar Abbas, Strait of Hormuz, Iran, reproductive ecology]; Bonyadi-Naeini et al. (2017: 2–6) [Abu-Musa Island, PG, Iran, name only]; Sánchez Ovando (2019: 45, Fig. 14A–C, 23) [ Dubai, the United Arab Emirates; description]; Lavajoo (2019: 93–97) [ Bandar Abbas, Strait of Hormuz, Iran, larval ecol- ogy]; Shabani et al. (2019: 1–9) [Northern coast of the Persian Gulf, ecology].
Spirobranchus cf. kraussii View in CoL .— Al-Kandari et al. (2019: 8) [ Kuwait, PG, name only, checklist of intertidal polychaetes of Ku- wait].
Galeolaria View in CoL sp.— Roohi-Shalmaee et al. (2019) [ Bandar Abbas, Strait of Hormuz, Iran, ecology].
Type material. Holotype: St.7, ZUTC.6780, on rock, high intertidal zone, legit S. Pazoki, 4A–B, Table 1 View TABLE 1 . Paratypes: total of 28 specimens, Fig. 1A View FIGURE 1 , Table 1 View TABLE 1 . Persian Gulf: St.1, ZUTC.6781 ( 1 spec.), ZUTC.6783 ( 1 spec.), ZUTC.6807 ( 1 spec.); St.2, ZUTC.6800 ( 1 juvenile), ZUTC.6806 ( 1 spec.); St.6, ZUTC.6779 ( 1 spec.); St.7, ZUTC.6782 ( 1 spec.), ZUTC.6805 ( 1 spec.); St.9, ZUTC.6784 ( 1 spec.), NHMO C7015 ( 1 spec.), ZUTC.6786 ( 1 spec.), ZUTC.6801 ( 1 juvenile); Strait of Hormuz: St.13, ZUTC.6796 ( 1 spec.), ZUTC.6797 ( 1 spec.); St.14, ZUTC.6791 ( 1 spec.); St.15, ZUTC.6788 ( 1 spec.), ZUTC.6789 ( 1 spec.), ZUTC.6799 ( 1 juvenile), ZUTC.6812 ( 1 juvenile), ZUTC.6813 ( 1 juvenile); St.17, ZUTC.6778 ( 1 spec.), NHMO C7016 ( 1 spec.), ZUTC.6802 ( 1 juvenile), ZUTC.6808 ( 1 spec.); St.18, AM W.52757 ( 1 spec.), ZUTC.6804 ( 1 spec. prepared for SEM); Gulf of Oman: St.22, ZUTC.6790 ( 1 spec.), AM W.52758 ( 1 spec.), legit. H. Ashrafi; St.25, ZUTC.6793 ( 1 spec.), ZUTC.6794 ( 1 spec.), ZUTC.6795 ( 1 spec.), ZUTC.6809 ( 1 spec.), ZUTC.6810 ( 1 spec.); all material legit by S. Pazoki from mid to high intertidal unless otherwise stated .
Material not included in the type series. Specimens with fringed peduncular wings: St.7, ZUTC.6815 ( 1 spec.); St.18, ZUTC.6816 ( 1 spec.); St.25, ZUTC.6814 ( 1 spec.); specimens with dissected talons: St.6, ZUTC. 6819 ( 1 spec.); St.14, ZUTC. 6820 ( 5 spec.); St.15, ZUTC.6818 ( 1 spec.), ZUTC. 6821 ( 7 spec.), St.17, ZUTC. 6822 ( 7 spec.), St.23, ZUTC. 6822 ( 7 spec.); St.25, ZUTC.6817 ( 1 spec.).
Material used for comparison. Spirobranchus kraussii, Danger Point, Walker Bay , South Africa, 34°29’S, 19°21’E, 10 Apr 2017, ZUTC.6803, legit H. van Niekerk ( 5 spec., one with opercular endplate dissected).
Description. Measurements are shown in the format of “mean ± SD [range/ holotype]” based on a holotype and 20 paratypes. The terminology follows that of ten Hove & Kupriyanova (2009).
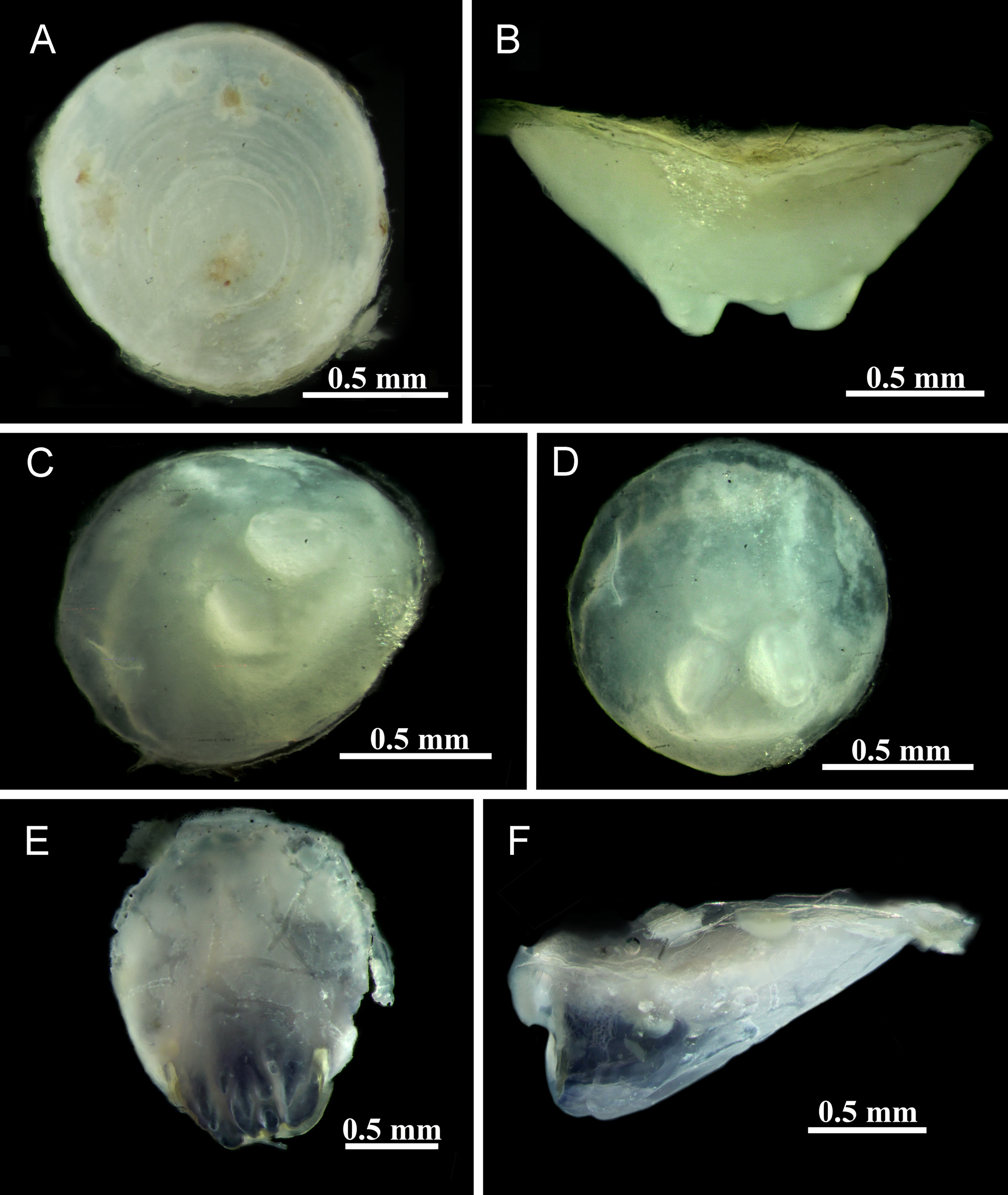
Tube trapezoidal or sub-triangular in cross section, 3.6 ± 0.8 mm [2.1–4.9/4.8] wide with a lumen of 1.8 ± 0.5 [0.9–3.0/2.5] mm in diameter and a projection from two longitudinal ridges expanding anteriorly making a flap over tube mouth ( Fig. 2C, D View FIGURE 2 ). In adults, flap expanded more laterally and anteriorly. However, juvenile tubes sometimes with a prominent wavy keel ( Fig. 2 View FIGURE 2 A–B). Outer surface of tube white, pale blue or violet (sometimes pink in small juveniles), inner surface white, blue or violet.
Radiolar crown composed of two radiolar lobes, each lobe with a mean of 16.6 ± 3.4 [12–20/20] radioles ar- ranged in a semicircle. Inter-radiolar membrane extending up to 48% ± 13% [30–66/59%] of radiolar length, usu- ally white, making a whitish band between the basal half of crown and free end of radioles. Prostomial eyes absent. Radiolar eyes in three to six pairs per radiole ( Fig. 4D View FIGURE 4 ). Stylodes absent. Radiolar crown violet, greenish blue, red, pink, yellow, and white ( Fig. 3 View FIGURE 3 A–D). Juveniles sometimes with lighter colours.
Peduncle triangular in cross-section, inserted near medial line between radiolar lobes ( Fig. 4C View FIGURE 4 ), distal part dorso-ventrally flattened; usually pigmented with dark and light bands, a total length of 1.9 ± 0.5 mm (1.1–2.6/ 1.8 mm). A transverse line making a two-segmented appearance of peduncle, 44.8 ± 6.2% (35–54/47%, Fig. 4 View FIGURE 4 B–C) from the proximal part of the peduncle. Peduncular wings with a smooth edge and a tapering tip ( Fig. 4 View FIGURE 4 B–C), but rarely fringed with 2–5 finger-like processes (seven specimens) from Dayyer (ZUTC.6815), Ramin (ZUTC.6814), and Hormuz (ZUTC.6816, Fig. 4D View FIGURE 4 ). Unpaired proximal wing absent. Pseudoperculum absent.
Operculum made of massive flattened ampulla covered with a white calcareous circular endplate ( Fig. 4 View FIGURE 4 A–D), 1.6 ± 0.4 mm (1.0–2.3/ 1.7 mm) in diameter, somewhat concave with a non-transparent rim, without any processes distally ( Figs 4 View FIGURE 4 C–D, 5A). Talon proximally inserted into tissue of opercular ampulla, thus usually not visible without dissection or clearing up tissues; talon consisting of two symmetrical dorsal or three arranged in a triangle dorsoventral protuberances, with a depression in between, where endplate meets tissue ( Fig. 5 View FIGURE 5 B–D).
Collar and thoracic membranes. Collar well-developed, sometimes as long as operculum ( Fig. 3A, C View FIGURE 3 ), with entire edge; trilobed, ventral lobe broader than dorso-lateral ones. Dorso-lateral lobes continuing in thoracic membranes forming an apron over first or second abdominal chaetigers. A pair of triangular tonguelets between lateral and ventral lobes present ( Fig. 4E View FIGURE 4 ). Collar creamy to dark blue or violet.
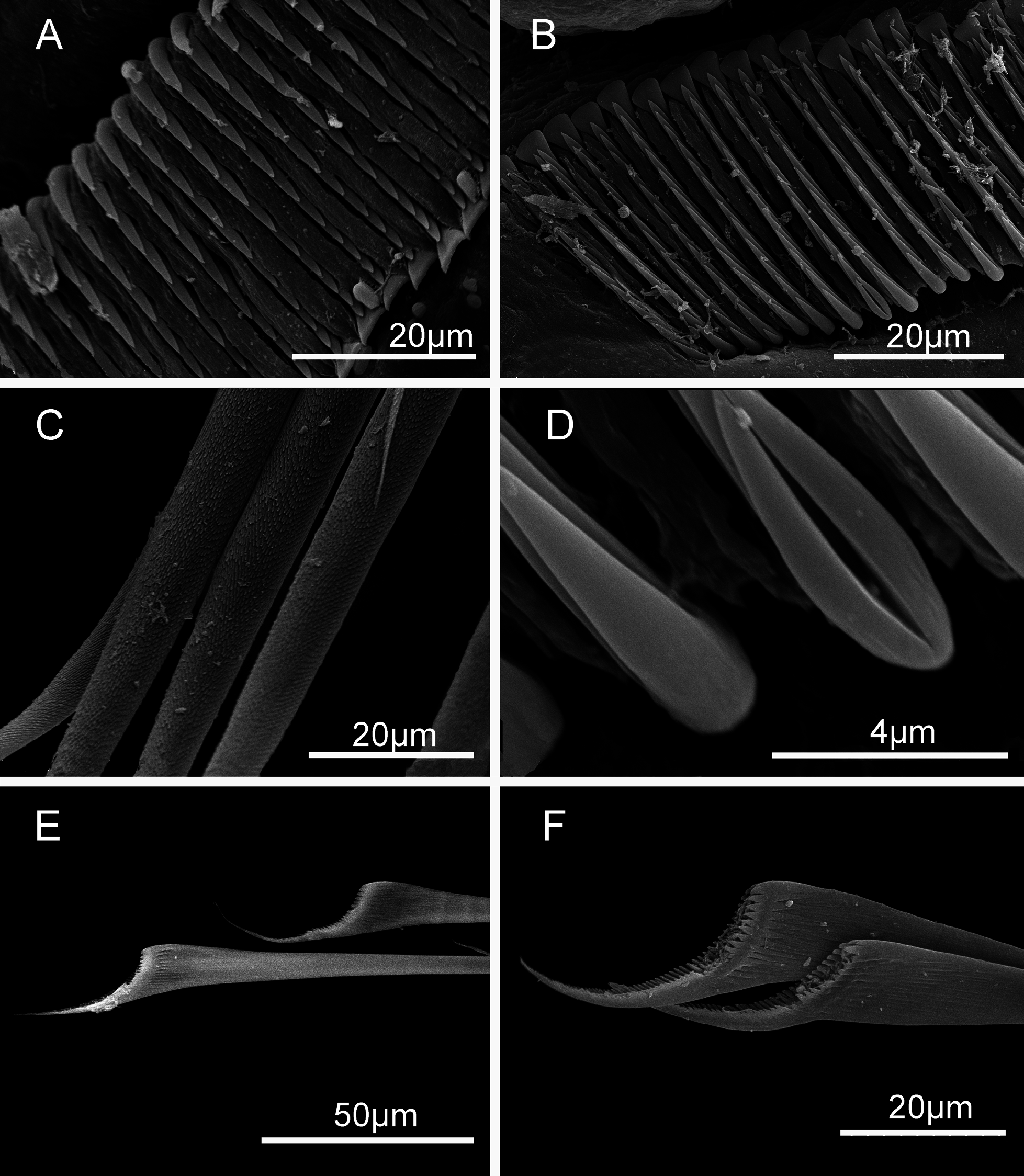
Thorax with six thoracic uncinigerous segments; collar chaetae absent in adults, but present in juveniles ( Fig. 4F View FIGURE 4 ). Thoracic chaetae limbate of two sizes ( Fig. 6C View FIGURE 6 ). Uncini saw-shaped with 7–10 teeth and flat gouged peg ( Fig. 6A View FIGURE 6 ). Thoracic tori similar in size along thorax, widely separated anteriorly, and gradually approaching posteriorly, forming a ventral depression ( Fig. 3 View FIGURE 3 B–D).
Abdomen. One or two achaetous segments anteriorly, number of chaetigers 41 ± 6 [31–50/38]. Abdominal chae- tae with long shaft present throughout abdomen ( Fig. 6E View FIGURE 6 ); distal end true trumpet-shaped, one side drawn out into a tapering process ( Fig. 6F View FIGURE 6 ). Uncini saw-shaped, similar to thoracic ones, with 8–12 teeth ( Fig. 6B, D View FIGURE 6 ) from anterior to posterior abdomen. Chaetae becoming increasingly longer posteriorly, but capillary chaeta absent in posterior chaetigers. Adults body proper brownish, yellow, red, green, but predominantly blue to violet ( Fig. 3 View FIGURE 3 ).
Size. Total length 8.6 ± 2.7 mm [4.8–15.4/ 8.8 mm]; width of thorax 1.8 ± 0.5 mm [1.0–3.3/ 1.6 mm]; radioles accounting for 22.0 ± 5.1% [11.9–29.4%] of entire length.
Habitat. Animals build large dense tube aggregations of 600,000 ± 15,000 specimens/m 2, with a maximum density occurring in summer ( Safari et al. 2014b) in the upper intertidal zone, attached to the upper surface of rocks ( Fig. 2 View FIGURE 2 F–G) or any hard substrates, including man-made structures, such as breakwater walls, concrete piers and rock jetties ( Table 2 View TABLE 2 ).
Type locality. Dayyer, Bushehr Province, Persian Gulf, Iran, 27°50’4.688”N, 51°57’0.143”E.
Geographic distribution. Persian Gulf: coasts of Iran, Kuwait, Saudi Arabia, the United Arab Emirates ( Abu Dhabi, Dubai); Gulf of Oman: Iran.
Reproduction and development. According to Crisp (1977), egg size in Spirobranchus sinuspersicus sp. nov. (as Pomatoleios kraussii ) is 60–65 µm and larvae show planktotrophic development typical for the genus ( Kupriyanova et al. 2001). Larvae complete their development to metamorphosis in 1–3 weeks at 25–27°C being fed by micro- flagellate Isochrysis galbana Parke or Tetraselmis suecica (Kylin) Butcher cultures under laboratory conditions. Competent larvae settle and metamorphose preferentially on the tubes of adults, especially on newly-settled ones ( Crisp 1977). In the field, larval settlement takes place from March to December with a maximum in August, which corresponds to water temperature of 30°C ( Mohammad 1975).
Taxonomic remarks. Specimens of Spirobranchus sinuspersicus sp. nov. described herein and S. kraussii from the type locality in South Africa are superficially similar in most of their morphological characters, such as the lack of collar chaetae in adults, shape of the tube and unadorned opercular endplate, as well in being gregarious and living in intertidal habitats. Nevertheless, these two species are morphologically recognizable.
The largest specimens of Spirobranchus sinuspersicus sp. nov. are noticeably smaller than those of S. kraussii 15 vs. 31 mm, respectively ( Fig. 3E View FIGURE 3 ). Differences in size are evident even in juvenile specimens ( 2.5–3.5 mm vs. 9.6–11.7 mm, respectively). Abdominal chaetigers are more numerous in S. kraussii than in S. sinuspersicus sp. nov. (70 ± 10 and 41 ± 6 segments, respectively). Pillai (2009), who had studied specimens of S. sinuspersicus sp. nov. (identified as Pomatoleios kraussii ) from Abu Dhabi, has reported the length of adult specimens to be 23.0 mm. However, among hundreds of specimens collected in the present study, none was even close to that size. Although Pillai (2009) did not mention the number of abdominal segments, based on his figure ( Pillai 2009, Fig. 49E, F), there are around 30 chaetigers.
Probably the most important morphological difference between Spirobranchus sinuspersicus sp. nov. and S. kraussii is the shape of the talon; a bulge made of two or three protuberances and a concavity between them in S. sinuspersicus sp. nov. ( Fig. 5 View FIGURE 5 A–D) and as an extension made of about 10 protrusions in S. kraussii ( Fig. 5 View FIGURE 5 E–F). This feature has been mentioned in specimens from Abu Dhabi ( Pillai 2009), but reported for 16 out of 18 specimens from Kuwait by ten Hove ( Simon et al. 2019). The major problem with this character is that its variability is not known.
Spirobranchus sinuspersicus sp. nov. also differs from S. kraussii by some more subtle characters. In S. sinuspersicus sp. nov. peduncular wings originate from the base of the peduncle resulting in a “V” shape appearance ( Fig. 4 View FIGURE 4 B–C), while in S. kraussii the wings originate from the mid-peduncle, resulting in a “Y” shaped appearance ( Fig. 3G View FIGURE 3 ). Furthermore, the opercular peduncle is located centrally between the radiolar lobes in S. sinuspersicus sp. nov. ( Fig. 4 View FIGURE 4 B–C), but it is inserted below left radiolar lobe in S. kraussii ( Fig. 3F View FIGURE 3 ; Simon et al. 2019). The abdominal chaetae in S. sinuspersicus sp. nov. are present from second or third abdominal segments, while they are present only in about 10 posterior abdominal segments of S. kraussii specimens.
The presence of fringed peduncular wings described herein have not been reported either for Spirobranchus kraussii or other for any species of the S. kraussii -complex, but such occasional presence might easily have been overlooked. Fringed wings are common for Spirobranchus species such as S. cf. tetraceros ( Ben-Eliahu & ten Hove 2011: 88, 93, Table 5) and S. cf. polytrema ( Phillippi, 1844) and populations of these taxa also have specimens with simple unfringed distal wings (ten Hove, pers. comm.). However, in S. sinuspersicus sp. nov., the wings are normally smooth, rarely fringed. Variability of this character, however, has been documented insufficiently in the literature.
Our morphological study also revealed the presence of occasional saw to rasp-shaped uncini in the new species. Some thoracic and abdominal uncini may have 2 or 3 teeth in one or two row(s) above peg in Spirobranchus sinuspersicus sp. nov. ( Fig. 6B View FIGURE 6 ), which was also seen in S. kraussii (unpublished SEM images by C. Simon, pers. comm.). Such uncini are also found in other species of Spirobranchus ( ten Hove & Kupriyanova 2009: 96) . Presence of occasional two teeth in the middle of a typical saw-shaped uncini ( Fig. 6D View FIGURE 6 ) is an unusual feature of S. sinuspersicus sp. nov. Additionally, the basal part of the posteriormost tooth of both thoracic and abdominal uncini maybe split in both S. sinuspersicus sp. nov. and S. kraussii ( Fig. 6B View FIGURE 6 and Simon’s unpublished photos).
Ecological observations. Spirobranchus kraussii inhabits the high to low intertidal zone, depending on wave exposure rate ( Simon et al. 2019). Aggregations of S. sinuspersicus sp. nov. were most abundant in high intertidal zones of sheltered to moderately exposed cliffs, on pebbles and rocks. They were hardly ever found in sub-tidal habitats, and if so, never in aggregations. Our observations are consistent with those of Crisp (1977) who reported preference of this species to settle on natural rather than artificial substrates. However, Mohammad (1975) reported S. sinuspersicus sp. nov. (as Pomatoleios kraussii ) from experimental settlement plates made of teak wood and some specimens were recently observed on PVC panels suspended 1 m deep for 3, 6 and 9 months in five Iranian ports ( Table 2 View TABLE 2 , St. 3, 8, 10, 11, 12, A. Nasrollahi, unpubl. data). Large aggregations were found on man-made structures, such as breakwater walls made of large stones ( Fig. 2G View FIGURE 2 ) and on cement wharf pilings and rock jetties in ports of Dayyer, Bushehr, Kong, and Jask. Shabani et al. (2019) reported the species (as S. kraussii ) on anthropogenic marine debris (e.g., plastic bottles, glass jars and wood) stranded on the coasts of the PG, arguing that this species can attach to various floating items regardless of the material type and surface texture. However, we did not find specimens of the new species either on floating substrates (such as buoys and ropes) or on hulls of small vessels such as ferries, fishing boats (open dories), and traditional dhows.
Spirobranchus sinuspersicus sp. nov. co-occurs with intertidal barnacles Amphibalanus amphitrite ( Darwin, 1854) and Chthamalus barnesi Achituv & Safriel, 1980 , as well as rock oysters Saccostrea cuccullata ( Born, 1778) . Spirobranchus sinuspersicus sp. nov. and C. barnesi make a distinct zone in the high intertidal, while A. amphitrite and S. cuccullata dominate lower intertidal where wave action is considerably strong (R. Naderloo, pers. comm.). According to Mohammad (1975), the bathymetric distribution of the worm extended between 0.4 and 1.9 m above datum (level below which the tide seldom falls) where specimens of S. sinuspersicus sp. nov. are limited by other encrusting species (e.g. ectoprocts, sponges and filamentous algae). In artificial bays, ports and on piers, S. sinuspersicus sp. nov. was relatively more abundant than either of the other three species.
Etymology. The specific epithet is an adjective and refers to the Persian Gulf (Sinus Persicus in Latin), the type locality of the new species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Spirobranchus sinuspersicus
| Pazoki, Samaneh, Rahimian, Hassan, Struck, Torsten H., Katouzian, Ahmad R. & Kupriyanova, Elena K. 2020 |
Spirobranchus maldivensis
| Pixell 1913 |
Galeolaria
| Lamarck 1818 |