Corymbia
|
publication ID |
https://doi.org/10.11646/zootaxa.2633.1.1 |
|
persistent identifier |
https://treatment.plazi.org/id/FB74996E-9E37-FFA0-ACA8-FF0EFAECD3D9 |
|
treatment provided by |
Felipe |
|
scientific name |
Corymbia |
| status |
|
Genus Corymbia View in CoL
Section Extensae
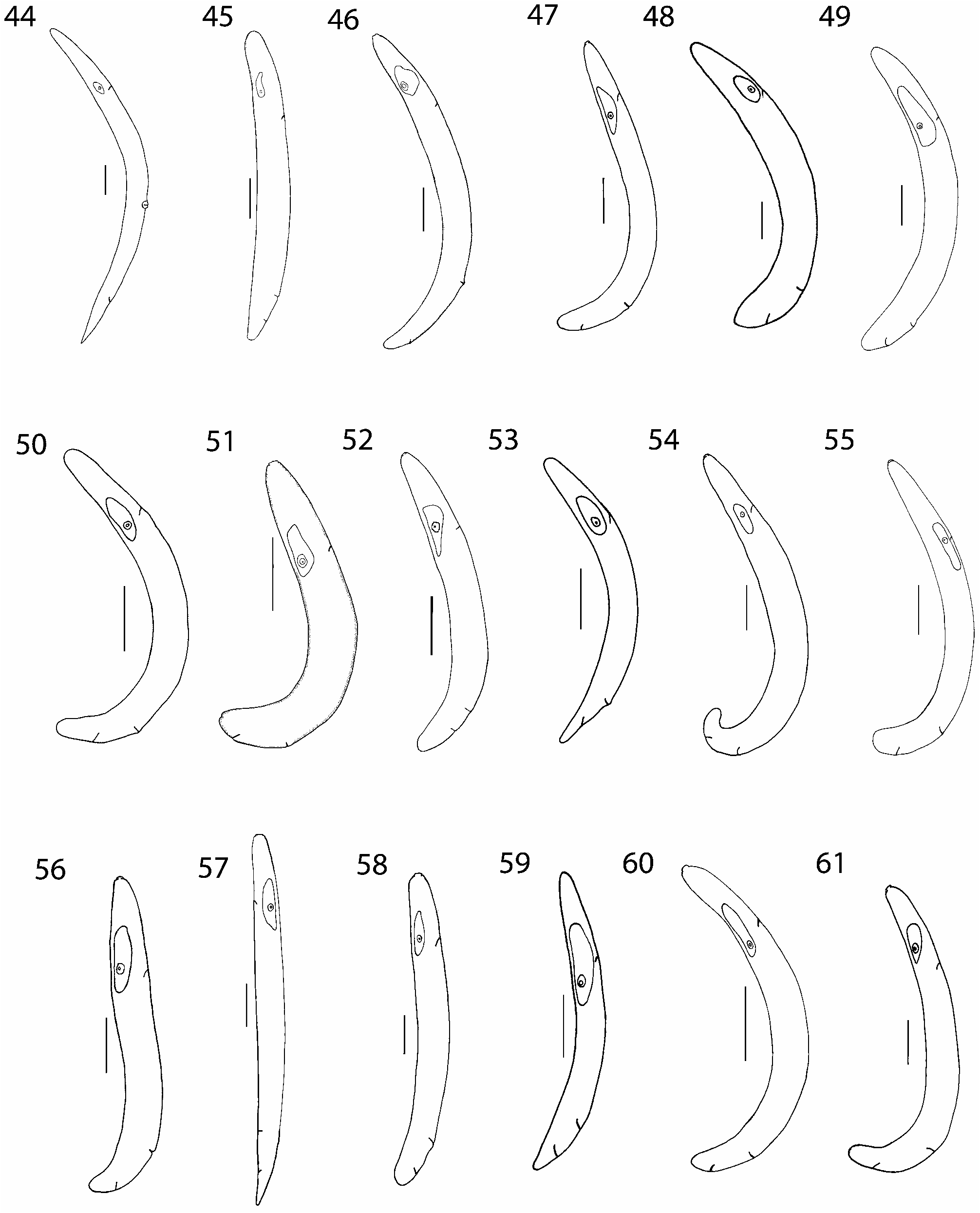
Fergusobia magna (voucher specimens nos 24, 42, 444, 327, 353, 452, 465); associated with an undescribed species of Fergusonina ( Figs 1 View FIGURES 1–22 , 23 View FIGURES 23–43 , 44 View FIGURES 44–61 ). Clade 1 in Fig. 78.
Form of gall. Terminal and axial bud ‘stem’ galls (various collections from Brisbane north to Cairns)
( Giblin-Davis et al. 2004a; Fig. 82 View FIGURES 80–88 ). Elliptical in shape, vary from less than 0.6 to more than 1.6 cm in diameter. Woody, with high numbers of locules (>100). Locules with lumens 0.8 to 1.12 mm in diameter in mature gall. Vascular bundles regularly spaced around the outer edges of the gall. Differs from other gall types examined histologically in that individual locules lacked the outer perimeter of red-staining cells.
Morphology of nematodes. Parthenogenetic female large, slender; variable to C-shape; small oesophageal gland; long slender conoid tail. Infective female large; slender, arcuate; small oesophageal gland; long slender conoid tail; V 51–62%. Male medium to large; slender, arcuate; small oesophageal gland; large angular spicule with anterior part longer than posterior; slender tail; bursa 30–50% ( Siddiqi 1986a; Davies et al. 2010).
Morphology of dorsal shield. (WINC 003284, 004382, 003241) ( Davies et al. 2010). Shield restricted to a broad area of weakly sclerotised spicules along the anterior margin of TS 2.
Possible relationships. Analyses of sequences from both D2/D3 and COI (Figs 78, 79) gave strong support (100%) for Clade 1 of Fergusobia . Intraspecific variation in D2/D3 sequences from differing collections suggested that populations of F. magna are genetically diverse, and may include a cryptic species ( Davies et al. 2010). Many locules within the galls coalesce, potentially allowing cross-fertilisation, which could increase genetic diversity if there are multiple fly foundresses ( Giblin-Davis et al. 2004a).
Section Septentrionales
Fergusobia ptychocarpae Davies 2008 (voucher specimen nos 30, 52, 450); associated with Fn. giblindavisi Taylor 2008 View in CoL
( Figs 12 View FIGURES 1–22 , 34 View FIGURES 23–43 , 54 View FIGURES 44–61 ). Clade 11 in Fig. 78.
Form of gall. Flower bud galls ( Giblin-Davis et al. 2004a, Taylor et al. 2005). Large (approximately 30 mm in diameter), each with several hundred larvae. Locules appear to originate from anther primordial cells and disc tissue at base of floret. Operculum dehisces, and mature buds frequently crack, allowing flies to escape.
Morphology of nematodes. Parthenogenetic female medium size; open C-shape; oesophageal glands large; tail narrow, conoid, bluntly rounded tip. Infective female medium to large; open C-shape or hooked behind vulva, cylindroid; short broad tail with broadly rounded tip. Male large size; arcuate to J-shape; medium oesophageal glands; angular spicule; tail relatively narrow, tip bluntly rounded; bursa 50–80% body length.
Morphology of dorsal shield. (WINC 003268). Shield restricted to a series of raised sclerotised spicules on the second and third thoracic segments ( Taylor & Davies 2008).
Possible relationships. Clade 11 comprised the flower bud galler from C. ptychocarpa , and a pea galler and flat leaf gallers from Eucalyptus spp. It was not strongly supported by analyses of sequences from D2/D3 (69%) (Fig. 78), and was not inferred from analyses of COI sequences (Fig. 79). However, in Ye et al. (2007b) the clade had 100% support in D2/D3 analyses. There are similarities in the shield form (reduced or missing) of the associated Fergusonina flies, and while the host plants come from sub-tropical areas of Australia they are genetically disparate. This may be an example of one fly lineage associated with two lineages of nematodes.
Unknown species of Corymbia
Fergusobia Morphospecies 3 (voucher specimen no 39); from Corymbia sp. associated with an unknown species of Fergusonina ( Figs 15 View FIGURES 1–22 , 37 View FIGURES 23–43 , 57 View FIGURES 44–61 ). Clade 14 in Fig. 78. Form of gall. Unilocular ‘pea’ (discrete) leaf galls on tiny leaflets, protruding from both sides of leaf blade ( Taylor et al. 2005) ( Fig. 87 View FIGURES 80–88 ).
Morphology of nematodes. Parthenogenetic female medium size, straight to arcuate shape; oesophageal gland medium; narrow conoid tail with narrowly rounded tip. Infective female small size; straight shape; relatively long slender conoid tail with narrowly rounded tip. Male medium size, almost straight shape; relatively long narrow tail; spicule arcuate; bursa ca 80% body length.
Morphology of dorsal shield. (WINC 003283). No shield or spicules.
Possible relationships. Clade 14 comprised Fergusobia from Corymbia sp. , a terminal leaf bud galler from E. near nitida , and pea gallers from E. marginata and E. pauciflora . Ye et al. (2007b) found strong support for this clade. In this work, analyses of sequences obtained from D2/D3 provided poor support for the clade as a whole (Fig. 78), but 100% bootstrap support for a clade comprising voucher specimens nos 286, 64 and 39. Voucher specimen no 39 was not sequenced for COI, but the other two vouchers grouped together in the phylogenetic tree inferred from it (Fig. 79).
Coming from a different host plant genus, the nematodes from Corymbia were from hosts genetically distant to those from Eucalyptus . While E. marginata , E. nitida and E. pauciflora are all classified as belonging to the subgenus Eucalyptus , they are placed in separate sections (respectively, Eucalyptus, Aromatica and Cineraceae). Thus, these hosts are not close genetically, and have widely disparate distributions (WA, TAS and eastern Australia). The associated nematodes have similar general form (but different sizes). The fly/nematode gall forms were of two types; and the fly larvae had two forms of dorsal shield. This may be an example of congruence of gall form for the pea gallers, or indicate a fly/nematode switch.
Genus Eucalyptus
Sub-Genus Eucalyptus
Section Amentum
Eucalyptus near acmenioides Schauer
Fergusobia voucher specimen no 340; associated with an unknown species of Fergusonina . Clade 12 in Fig. 78.
Form of gall. Terminal (occasionally axilliary) shoot bud galls ( Giblin-Davis et al. 2004a; Taylor et al. 2005). Broadly ovate galls, containing ca 10 to 100 locules.
Morphology of nematodes. Parthenogenetic female small to medium size; variable shape; oesophageal glands medium; tail relatively narrow, conoid, tip bluntly rounded. Infective femal medium; arcuate to open C-shape; short broad tail with hemispherical tail tip. Male medium size; variable shape; medium oesophageal glands; strong angular spicule; bursa 70–80% body length.
Morphology of dorsal shield. (WINC 004200). Fly larvae not available. Puparia lacking shield. Note that because cyclorraphan fly puparia retain the cuticle of the last molt, the puparia have the characteristics of the L 3 larvae, and can be used to determine shield form .
Possible relationships. These nematodes apparently clump (Clade 12) with those sequenced from Angophora (respectively, Morphospecies 1 and 2). See discussion under A. floribunda .
Section Aromatica
Series Insulanae
Fergusobia voucher specimen no 276; associated with an unknown species of Fergusonina . Clade 6 in Fig. 78.
Form of gall. Terminal leaf bud gall. Similar morphology to that of E. delegatensis , but not glaucous.
Morphology of nematodes. Parthenogenetic female medium in size, arcuate to open C-shape; oesophageal gland large; tail short, conoid, relatively slender, bluntly rounded tip. Infective female arcuate to open Cshape, maximum width at mid-length; tail short, broad, tip almost hemispherical, V ca 80–90%. Male arcuate shape; tail ventrally concave, tip bluntly rounded; spicules angular; bursa ca 60% body length.
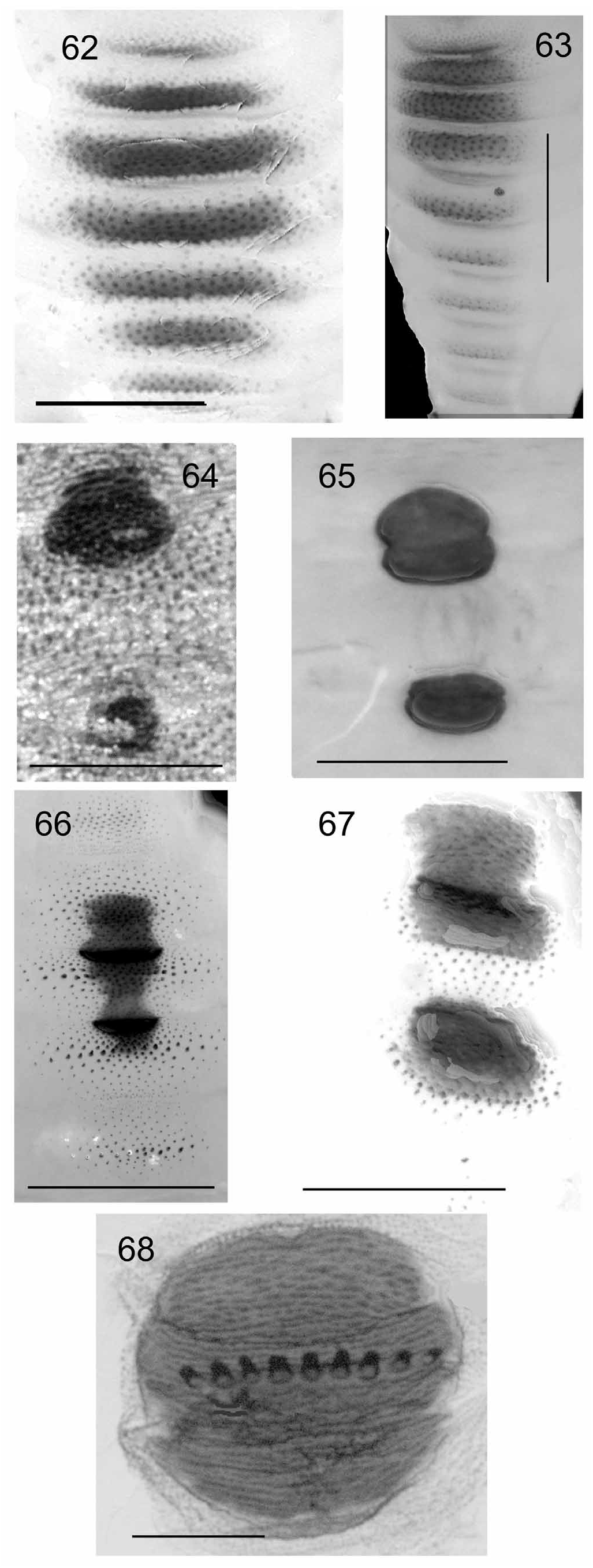
Morphology of dorsal shield. (WINC 003463). Shield comprising six transverse ‘bars’ of narrow patches of small spots of sclerotised cuticle ( Fig. 62 View FIGURES 62–68 ).
Possible relationships. Clade 6 (Fig. 78) comprised Fergusobia nematodes from TLGs from E. amygdalina , E. delegatensis , E. diversifolia , E. racemosa , and E. sp., FLGs from E. sp., and FBGs from E. macrorhyncha and E. obliqua . Ye et al. (2007b) found strong support for this clade. In this work, analyses of sequences obtained from D2/D3 provided poor support for Clade 6 as a whole (Fig. 78), but 93% for a grouping comprising voucher specimens nos 276, 281, 284, 33, 36, 7, 41, and 337. With COI, nematodes from TLG’s from E. diversifolia , E. delegatensis and E. racemosa grouped together in the phylogenetic tree inferred from the sequences (Fig. 79).
All the host species in this clade are placed in subgenus Eucalyptus , but from five different sections, i.e., they are genetically related but not closely. Of the known species, only E. racemosa occurs in northern Australia.
E. near nitida Hook.
Fergusobia voucher specimen no 286; associated with an unknown species of Fergusonina . Clade 14 in Fig. 78.
Form of gall. Shoot bud galls similar to those described from E. diversifolia .
Morphology of nematodes. Parthenogenetic female medium size; arcuate in shape; oesophageal gland large; conoid tails. Male medium size; arcuate shape; oesophageal gland medium; spicule moderately angular (at less than 90°), moderately sclerotised; bursa arising at ca 50% body length.
Morphology of dorsal shield. No associated fly larval material collected.
Possible relationships. Grouped in Clade 14; see discussion under E. amygdalina .
Series Pachyphloiae
Fergusobia Morphospecies 4 (voucher specimens nos 4, 350); associated with Fn. nicholsoni . Clade 6 in Fig. 78.
Form of gall. Flower bud galls ( Currie 1937, Giblin-Davis et al. 2004a, Taylor et al. 2005). Galls clubshaped, larger than uninfested flower buds. Locules originate in a proliferation of anther cells, are membranebound, and each is attached to floret wall by an anther filament. Filaments in infested buds 2 to 3 times the diameter of those of uninfested buds. Stigma not apparent.
Morphology of nematodes. Parthenogenetic female small to medium in size, arcuate to C-shape; oesophageal gland large; tail short, broadly conoid, broadly rounded tip. Infective female J-shape, maximum body width at mid-length; narrows gradually behind vulva; tail tip bluntly rounded, V ca 80%. Male J-shaped; bursa 20 – 40% body length; spicules angular; tail ventrally concave, short, tip bluntly rounded.
Morphology of dorsal shield. Associated with Fn. nicholsoni (WINC 003118). Broad, heavily sclerotised cuticular plates. First a very short, broad sclerotised plate on posterior edge of TS 3, confluent with a broader sclerotised plate on AS 1, and a slightly narrower plate on the anterior margin of AS 2. The ‘bar’ form of dorsal shield is illustrated in Figs 62 and 63 View FIGURES 62–68 .
Possible relationships. Grouped in Clade 6; see discussion under E. amygdalina . From D2/D3 sequencing, there appeared to be little or no genetic drift between populations of Morphospecies 4 collected from disjunct populations of E. macrorhyncha in SA and the ACT (W. Ye, unpub. data). However, sequences from COI did suggest drift (Fig. 79).
The shield form of the flies from E. macrorhyncha (plates) as compared with others in this clade (bars) suggests that the flies are from different lineages. The shield is similar to that of fly larvae from FBG on E. obliqua . Nematodes from E. macrorhyncha may belong to a separate clade.
Series Pachyphloiae
E. eugenioides Sieber ex Sprengel
Fergusobia Morphospecies 5 (voucher specimen no 349); associated with an unknown species of Fergusonina . Clade 13
in Fig. 78.
Form of gall. Flower bud galls ( Fig. 83 View FIGURES 80–88 ).
Morphology of nematodes. Parthenogenetic female small to medium size; open C to arcuate shape; large oesophageal glands; short conoid tail with bluntly rounded tip. Infective female medium size; arcuate with hooked tail; cylindroid; broad tail with broadly rounded tip; V 83%. Male medium to large; almost straight to J-shape; medium to large oesophageal gland; short tail with bluntly rounded tip; weak angular spicules, bursa ca 25%.
Morphology of dorsal shield. (WINC 004221). Shield comprising 3 broad confluent plates of moderately sclerotised cuticle on TS 3, AS 1 and AS 2 respectively; no teeth.
Possible relationships. Clade 13 comprised Fergusobia nematodes from FLGs on E. spp. voucher specimen nos 29 and 348, E. microcarpa , E. leucoxylon , E. siderophloia , and E. porosa , and PGs on E. porosa and E. sp. voucher specimen no 351. Analyses of sequences of D2/D 3 in this work (Fig. 78) provided 94% support for this clade, which received 100% support in Ye et al. (2007b). Analyses of sequences from COI gave the grouping 80% support (Fig. 79), and it included voucher specimen no 2 from FBG on E. obliqua , but not voucher specimen no 349 from FBG on E. eugenioides .
While E. eugenioides is classified as subgenus Eucalyptus , the other host eucalypts in this clade are from subgenus Symphyomyrtus , i.e., are genetically disparate. The distribution of E. eugenioides overlaps that of E. microcarpa and E. siderophloia ; and distributions of E. microcarpa , E. leucoxylon and E. porosa also overlap. Host switching between E. eugenioides and microcarpa and siderophloia may have occurred.
The gall form of voucher specimen no 349 from E. eugenioides (FBG) differed from that of the other members of this clade (PG and two forms of FLGs) suggesting that the associated flies had evolved different preferences for oviposition sites. If gall form reflects the particular meristem selected by a female fly for egg deposition, this could explain the occurrence of similar gall forms in widely disparate clades, and apparent convergence.
Section Eucalyptus
Fergusobia voucher specimen no 64; associated with an undescribed species of Fergusonina . Clade 14 in Fig. 78.
Form of gall. Unilocular ‘pea’ (discrete) leaf galls ( Taylor et al. 2005) ( Fig. 87 View FIGURES 80–88 ). Hemispherical, protruding only from the top surface of the leaf blade. Each 5–6 mm in diameter, with a height of 3–5 mm. Neighboring galls do not merge.
Morphology of nematodes. Parthenogenetic female large in size; straight shape; oesophageal gland medium; slender conoid tails. Male large size; arcuate shape; oesophageal gland medium; spicule moderately angular (at less than 90°), moderately sclerotised; bursa arising at ca 90% body length.
Morphology of dorsal shield. (WINC 003434, 003438). No dorsal shield.
Possible relationships. Grouped in Clade 14; see discussion under Corymbia sp. for comments.
While E. marginata , E. nitida and E. pauciflora are all classified as belonging to the subgenus Eucalyptus , they are placed in separate sections (respectively, Eucalyptus, Aromatica and Cineraceae). Thus, these hosts are not close genetically, and have widely disparate distributions (WA, TAS and eastern Australia). However, fly shield morphology is similar, and the associated nematodes have similar general form (but different sizes). This suggests that the fly/nematode association developed from a common lineage before the sea incursion that separated WA from eastern Australia from the late Eocene to the mid-Miocene ( Nelson, 1981).
Series Eucalyptus
Fergusobia voucher specimens nos 2, 315; associated with an undescribed species of Fergusonina (respectively, Figs 31 View FIGURES 23–43 ,
70 View FIGURES 69–72 , 32 View FIGURES 23–43 , 71 View FIGURES 69–72 ). Clade 6 in Fig. 78.
Form of gall. Flower bud galls, similar in form to those on E. macrorhyncha ( Giblin-Davis et al. 2004a) .
Locules develop from proliferation of anther cells. Each locule attached to floret wall by an anther filament.
Stigma does develop in these galls.
Morphology of nematodes. Parthenogenetic female (voucher specimen no 2, WNC 2019) medium size, open C or C-shape; oesophageal glands large; tail short, conoid, tip narrowly rounded. Infective female Jshape, maximum body width at mid-length or at vulva; tail tip broadly rounded or almost hemispherical. Male J-shaped; spicules angular; tail tip bluntly rounded; bursa 20 – 40% body length.
Parthenogenetic female (voucher specimen no 315, WNC 2228) as above, except that in these specimens the tail is sub-cylindroid and the tail tip is bluntly rounded.
Morphology of dorsal shield. (WINC 003450, 003470). Shield comprising broad, heavily sclerotised cuticular plates. First a very short, broad sclerotised plate on posterior edge of TS 3, confluent with a broader sclerotised plate on AS 1, and a slightly narrower plate on the anterior margin of AS 2. Shield form similar to that of Fn. nicholsoni .
Possible relationships. From sequencing of D2/D3, these nematodes were inferred to belong to Clade 6. Grouped in Clade 6; see discussion under E. amygdalina for comments. Sequences from nematodes from FBG on E. obliqua appear in two different groupings in the COI analyses (Fig. 79). These samples came from two trees at one site in SA, and may a) represent an artifact, or b) the occurrence of multiple fly foundresses carrying genetically disparate Fergusobia nematodes, or c) the presence of two nematode/fly species with similar gall type on the one species of host plant.
Parthenogenetic females sequenced from FBG on E. obliqua had two distinct morphotypes, from two respective sites. This suggests that two different lineages of nematodes and flies have utilised one form of meristematic tissue on E. obliqua , and emphasises the need for care when collecting fly/nematode galls.
Nematodes from FBG from E. macrorhyncha and obliqua are morphologically similar. While both host plant species are from subgenus Eucalyptus , they are classified in different sections (respectively, Capillulus and Eucalyptus ), i.e., they are not genetically close. Host plant distributions overlap in NSW. Galls on each host species were similar. The associated fly larvae had similar shields with three confluent plates of heavily sclerotised cuticle of which the middle was the widest, and no teeth. This is a possible case of a common ancestral lineage, and host switching. Further investigation is needed to differentiate the two morphotypes of Fergusobia collected from these FBG, and to elucidate their relationships.
Section Insolitae
Fergusobia Morphospecies 6 (voucher specimen no 275); associated with an undescribed species of Fergusonina (Figs
9, 31, 52). Clade 8 in Fig. 78.
Form of gall. ‘Leafy’ leaf bud galls ( Taylor et al. 2005), consisting of fused gall tissue, the locules held in a single plane by expanding leaf stem and leaf tissue, so that leaf and meristematic stem tissue covers an elongate, chilli-shaped gall, usually with leaf tissue growing beyond it.
Morphology of nematodes. Parthenogenetic female small to medium size, open C-shape; large oesophageal gland; body narrows rapidly behind vulva; short conoid tail with narrowly or bluntly rounded tail tip. Infective female arcuate, cylindroid; hemispherical tail tip. Male medium size, arcuate or J-shape; oesophageal glands medium; angular spicule; bursa arises near secretory/excretory pore.
Morphology of dorsal shield. (WINC 003065). Shield comprising 9 transverse ‘bars’, the first on TS 2, followed by three broader bars on TS 3 and AS 1–2, then a narrower bar on AS 3. These bars are formed from patches of raised, heavily sclerotised cuticle, dotted with raised sclerotised spicules. The patches are surrounded with areas of raised, sclerotised, sparse spicules. The following bars are progressively reduced to rows of raised sclerotised spicules on AS 4–7.
Possible relationships. Clade 8 comprises these nematodes from E. planchoniana and nematodes from pea galls on E. leucoxylon . Analyses of sequences from D2/D3 provided weak (77%) support for this clade (Fig. 78). Unfortunately, nematodes from E. planchoniana were not sequenced for COI.
Genetically, E. planchoniana and E. leucoxylon are widely disparate hosts (coming from subgenera Eucalyptus and Symphyomyrtus respectively), and their geographic ranges do not overlap. Morphology of the galls, fly larval shields and respective Fergusobia is different. Thus, this seems an unlikely clade. Sequences from E. planchoniana were not included in the COI tree, where Fergusobia sp. voucher specimen no 71 appeared with Eucalyptus sp. voucher specimen no 32 ( Figure 3 View FIGURES 1–22 in Ye et al. 2007b).
Section Cineraceae
Series Fraxinales
Fergusobia Morphospecies 7 (voucher specimen no 281); associated with an undescribed species of Fergusonina ( Figs 6 View FIGURES 1–22 , 28 View FIGURES 23–43 , 49 View FIGURES 44–61 ). Clade 6 in Fig. 78.
Form of gall. Terminal and axial leaf bud galls similar to those from the subgenus Symphyomyrtus , but less spheroid, more ‘chilli-shaped’, multilocular, and often with leaf tissue protruding/growing from them.
Glaucous.
Morphology of nematodes. Parthenogenetic female medium to large in size, arcuate to open C-shape;
oesophageal glands large; tail short, conoid, bluntly rounded tip. Infective female arcuate, cylindroid; short broad tail, tip almost hemispherical; V 80–90%. Male shape variable; tail tip broadly rounded; spicules strong,
angular; bursa arises near secretory/excretory pore.
Morphology of dorsal shield. (WINC 003478-9). Shield comprises 5 transverse ‘bars’ of raised sclerotised spicules. The first, on TS 3, consists of a short, broad, transverse area of raised sclerotised spicules; the second, is shorter and broader on AS 1; next three progressively reduce in prominence from AS 2–4.
Possible relationships. Grouped in Clade 6; see discussion under E. amygdalina .
Series Pauciflorae
E. pauciflora Sieber ex Sprengel
Fergusobia voucher specimen no 280; associated with an undescribed species of Fergusonina . Clade 14 in Fig. 78.
Form of gall. Unilocular ‘pea’ (discrete) leaf galls ( Taylor et al. 2005) ( Fig. 87 View FIGURES 80–88 ). Hemispherical, protruding only from the top surface of the leaf blade. Neighboring galls do not merge.
Morphology of nematodes. Parthenogenetic female medium to large size; arcuate to straight shape; oesophageal glands relatively small; slender conoid tails. Male medium to large size; arcuate or straight shape; oesophageal glands small or medium; spicule moderately angular (at more than 90°), moderately sclerotised; bursa arising near secretory/excretory pore.
Morphology of dorsal shield. (WINC 003475). Larvae not seen. Pupa has no apparent dorsal shield.
Possible relationships. Grouped in Clade 14; see discussion under Corymbia sp.
Series Psathroxylon
Fergusobia voucher specimens nos 41, 337; associated with an undescribed species of Fergusonina . Clade 6 in Fig. 78.
Form of gall. Terminal leaf bud galls ( Taylor et al. 2005). Similar morphology to that of E. delegatensis , but not glaucous.
Morphology of nematodes. Parthenogenetic female medium size, C-shape; oesophageal glands large; tail short, conoid, bluntly rounded tip. Male arcuate to J-shaped; oesophageal glands medium; spicules angular; tail tip bluntly rounded; bursa ca 50% body length.
Morphology of dorsal shield. (WINC 003075). No larval material available. Pupal shield comprising 6 broad bars of sclerotised, raised spicules. The first is in the posterior half of TS 2, and the second and third on AS 1 and 2, respectively. The bars of raised spicules become progressively less prominent and sparser on AS 3 and 4.
Possible relationships. Grouped in Clade 6; see discussion under E. amygdalina .
The distribution of E. racemosa (in the north-east of Australia) is disjunct from that of E. amygdalina , E. delegatensis and E. diversifolia (in the south-east of Australia), all of which have similar galls with flies with similar ‘barred’ dorsal shields. This suggests that the fly/nematode mutualisms found on these hosts developed before the separation of TAS from the Australian mainland, the lineage was widespread, and that hostswitching occurred. These hosts are all classified as subgenus Eucalyptus , i.e., have some genetic similarity.
Section Longistylus
Fergusobia Morphospecies 8 (voucher specimens nos 7, 284); associated with an undescribed species of Fergusonina .
Clade 6 in Fig. 78.
Form of gall. Terminal and axial leaf bud galls (Giblin-Davis 2004, Taylor et al. 2005). Elongate, ‘chillishaped’, multilocular. Fresh galls comprised rigid but relatively soft plant tissue. Usually have leaf tissue protruding/growing from the tip. When sectioned and stained, outer layers of cells in the gall stained red, contained scattered oil glands. Matrix comprised vacuolated parenchymal cells. Lumen of locules lined with granular looking hypertrophied cells, 2–6 cell layers deep.
Morphology of nematodes. Parthenogenetic female medium to large in size, variable shape; oesophageal glands large; tail short, conoid, tip bluntly rounded. Infective female large, arcuate shape; maximum width at mid-length; tail short, broad, tip bluntly rounded or almost hemispherical; V 80–90%. Male medium to large size, arcuate to J-shaped; spicule angular; tail tip bluntly rounded; bursa arises near secretory/excretory pore.
Morphology of dorsal shield. (WINC 002985-7, 002992-3). Shield with 8 or 9 ‘bars’ of raised, sclerotised spicules. First bar on TS 2, short, narrow, towards posterior margin; second on TS 3, longer and broader; third on AS 1, longer and broader again. The bars of raised sclerotised spicules decreased in prominence from AS 1–5. Spicules sparse to almost absent on AS 6 and 7.
Possible relationships. Grouped in Clade 6; see discussion under E. amygdalina and E. racemosa .
Vouchers specimens nos 284 and 7 from E. diversifolia appear to be the same genetically with COI sequences, but different with D2/D3 sequences. This may be an artifact, or there may have been multiple fly foundresses for this gall form.
Subgenus Symphyomyrtus
Section Sejunctae
a) Unilocular leaf ‘pea’ gall
Fergusobia voucher specimen no 740; associated with an unknown species of Fergusonina . No sequences for D2/D3.
Form of gall. Unilocular stem and bud galls at Meningie SA; similar in form to those on E. gomphocephala .
Morphology of nematodes. Parthenogenetic female small, C-shape; oesophageal gland large; body narrows gradually behind vulva; tail conoid, tip bluntly rounded. Male medium size, J-shape; oesophageal gland medium; tail tip bluntly rounded; spicule angular; bursa ca 40% body length.
Morphology of dorsal shield. (WINC 063703). Shield comprises heavily sclerotised plates, with teeth. A heavily sclerotised broad plate on the posterior margin of TS 3, confluent with a broad heavily sclerotised plate on AS 1 and a shorter, heavily sclerotised broad plate on the anterior margin of AS 2. A row of 4–5 distinctive, short, sharply pointed, forward projecting teeth arises from the posterior edge of the plate on AS 1.
Possible relationships. These nematodes were not sequenced for D2/D3. With COI (Fig. 79), they form part of a grouping which included pea gallers from E. leucoxylon and E. sp. voucher specimen no 32, and axial bud gallers from E. leucoxylon voucher specimen no 741. The distributions of the host species overlap, and all are classified as subgenus Symphyomyrtus , but their relationships are unclear because E. cladocalyx is unplaced. While similar in that the shield is of the ‘plates with teeth’ form, the dorsal shield of these fly larvae is distinctive and suggests that the flies are from a different lineage to that of the Fergusonina inducing TLG on other host plants from the subgenus Symphyomyrtus , e.g., E. camaldulensis . Morphology of the various fergusobid nematodes is similar, but body size and length of the bursa in the males differ.
b) Terminal leaf bud gall
Fergusobia voucher specimen no 739; associated with an unknown species of Fergusonina . No sequences for D2/D3.
Form of gall. Small terminal leaf bud gall, containing 4 or 5 locules.
Morphology of nematodes. Parthenogenetic female small, open C-shape; oesophageal gland medium; body narrows gradually behind vulva; short broad tail, tip broadly rounded. Infective female small size, open C-shape; short broad tail, broadly rounded tip; V ca 85%. Male medium size, arcuate to J-shape; oesophageal gland medium; tail tip bluntly rounded; spicule angular; bursa 20–30% body length.
Morphology of dorsal shield. (WINC 063702). Shield comprising two minute patches of sclerotised cuticle, the first made up of a small spot on the posterior margin of TS 3 confluent with a small spot on the anterior margin of AS 1, and the second made up of a band of sclerotised cuticle on the posterior margin of AS 1 confluent with a small spot of sclerotised cuticle on the anterior margin of AS 2. A few sparse sclerotised spicules occur behind the first and second spots respectively.
Possible relationships. These nematodes did not sequence for D2/D3. With COI (Fig. 79), they were inferred to form part of a group which included flat leaf gallers from E. porosa and E. leucoxylon . All nematodes were associated with fly larvae having shields comprising two sclerotised patches. Morphology of the nematodes was also similar. The distributions of the host species overlap, and all are classified as subgenus Symphyomyrtus , but their relationships are unclear because E. cladocalyx is unplaced. Host switching could have occurred.
Section Bolites
Fergusobia Morphospecies 9 (voucher specimen no 63); associated with Fn. newmani Tonnoir 1937 View in CoL ( Figs 8 View FIGURES 1–22 , 30 View FIGURES 23–43 , 51 View FIGURES 44–61 ).
Clade 7 in Fig. 78.
Form of gall. Small pea-like unilocular galls on young stems and leaf buds ( Currie 1937; Taylor et al. 2005) ( Fig. 88 View FIGURES 80–88 ). Consist of discrete chambers 2–3 mm in diameter, and about 2 mm in height. Hemispherical, protrude only from one surface of a newly expanded leaf.
Morphology of nematodes. Parthenogenetic female small to medium size, C-shape; oesophageal gland enormous; body narrows rapidly behind vulva; short conoid tail with bluntly rounded tip. Infective female small, variable shape; maximum width at vulva; short broad tail; V 70–85%. Male small, arcuate or J-shaped; oesophageal gland enormous; spicule angular; short tail with broad tip; bursa 20–30% body length.
Morphology of dorsal shield. Fn. newmani (WINC 003426-7). Shield comprises a heavily sclerotised plate on the posterior margin of TS 3, confluent with heavily sclerotised plate on AS 1, and a short broad plate on anterior margin of AS 2. Two long, widely spaced, anterior projecting, recurved, sharp teeth arise from the posterior margin of AS 1.
Possible relationships. Clade 7 (Fig. 78) comprised these Fergusobia from E. gomphocephala and pea gallers from E. sp. voucher specimen no 32 and E. leucoxylon and flat leaf gallers from E. leucoxylon . Analyses of sequences from D2/D3 gave weak (75%) support for Clade 7 (Fig. 78). Nematodes from this clade are morphologically similar, and are associated with fly larvae with similar forms of dorsal shield.
Except for E. gomphocephala (Section Bolites), host plants in this clade are genetically similar (Section Adnataria), suggesting that host switches could have occurred. Eucalyptus gomphocephala has a disjunct distribution from the other host species in this clade. This suggests that the fly/nematode association collected from E. gomphocephala developed from a common lineage before the sea incursion that separated WA from eastern Australia from the late Eocene to the mid-Miocene ( Nelson 1981).
Section Exsertaria
Series Erythroxylon
(a) Leaf ‘pea’ galls Fergusobia (voucher specimen no 34), associated with an unknown species of Fergusonina ( Figs 10 View FIGURES 1–22 , 32 View FIGURES 23–43 ). Clade 9 in Fig. 78.
Form of gall. Leaf ‘pea’ galls ca 2–3mm in diameter, comprising a single chamber. Often found at the tip of a young expanding leaf.
Morphology of nematodes. Parthenogenetic female medium size, open C-shape; oesophageal gland large;
short conical tail. Male small to medium size, straight with tail arcuate; oesophageal gland medium; tail tip broadly rounded; angular spicule; bursa ca 20% body length.
Morphology of dorsal shield. (WINC 003105). No shield or spicules.
Possible relationships. Clade 9 included these nematodes and a pea galler from E. sp. voucher specimen no 31. Here, there was little support for the clade from sequences of D2/D3. From sequencing of D2/D3, Ye et al. (2007b) obtained strong support (100%) for the clade. In the present work, analyses of sequences of COI also supported the grouping (Fig. 79; 100% support). It was also supported by the lack of a shield in the associated Fergusonina fly larvae, and morphological similarity of the nematodes collected.
Eucalyptus sp. (Section Adnataria) and E. tereticornis (Section Exsertaria) are not close genetically, but their distributions overlap (both samples were collected in Brisbane). This suggests that host-switching by the fly/nematode mutualism could have occurred.
(b) Terminal leaf bud gall
Fergusobia (voucher specimens nos 401, 438) associated with an undescribed species of Fergusonina . Clade 3 in Fig. 78.
Fergusobia from E. sp. near tereticornis (voucher specimen no 446) associated with an unknown species of Fergusonina . Clade 3 in Fig. 78.
Form of gall. Terminal leaf bud galls on E. tereticornis ; similar in form to those from E. camaldulensis
(K.A. Davies, unpub. data).
Morphology of nematodes. Parthenogenetic female of medium size, C-shape; body narrows rapidly behind vulva; large oesophageal gland; conoid tail. Infectives larger than parthenogenetic female, arcuate,
cylindroid; short tail with bluntly rounded or hemispherical tip; vulva at 64–80%. Male medium size, straight to J-shape, oesophageal gland medium to large, angular spicule; bursa 12–40%, tail relatively slender with rounded tip or broad with broadly rounded tip.
Morphology of dorsal shield. (WINC 004388). Similar to that of fly larvae from E. camaldulensis TLG.
Shield comprising three confluent, sclerotised plates extending over three segments (TS 3, AS 1 and AS 2).
Surrounded by many sclerotised raised spicules, arranged in whorls. Seven small teeth arise from the middle plate, projecting forward. There is also a row of raised spicules running transversely across the fourth abdominal segment.
Possible relationships. Clade 3 comprised nematodes forming TLG from E. tereticornis and E. camaldulensis . In this work, the clade was well supported (87%) for D2/D3 sequences, and also in the analyses of Ye et al. (2007b) (100%). With COI, the inferred grouping had 97% support.
Based on similar morphology of nematodes, fly larvae and gall form, it is likely that nematodes from several host plant species, all from subgenus Symphyomyrtus , belong to this putative clade (K.A. Davies, unpub.
data). Genetically, E. tereticornis and E. camaldulensis are similar (from Section Exsertaria). Their distributions overlap. Fly larvae from E. camaldulensis and E. tereticornis have similar shield types, with sclerotised plates with teeth. Nematodes from galls on each are also of similar morphological type and have similar molecular sequences. Given the genetic similarity and overlapping distributions of E. camaldulensis and E.
tereticornis, this may be a case of coevolution of the fly/nematode mutualism and the host plant.
Collection of both a pea-galling (voucher specimen no 34) and a terminal leaf bud galling (voucher specimen no 438) fly/nematode mutualism from E. tereticornis , from different genetic clades, showed that at least two distinct lineages of the mutualism develop on this one host.
Series Rostratae
(a) Terminal and axial shoot bud galls
Fergusobia brittenae Davies 2010 (voucher specimens nos 62, 205, 292, 59, 55); associated with Fn. lockharti Tonnoir View in CoL
1937 ( Figs 3 View FIGURES 1–22 , 25 View FIGURES 23–43 , 46 View FIGURES 44–61 ). Clade 3 in Fig. 78.
Form of gall. Terminal and axial shoot bud galls ( Fig. 84 View FIGURES 80–88 ) ( Giblin-Davis et al. 2004a, Taylor et al. 2005). Entire bud becomes a fused spheroidal mass of tissue. Developing leaves entirely fused in some; in others part grows on separately from gall. Ovate, 0.5 to 3 cm in diameter. No distinct demarcation between locule and gall matrix. Locules surrounded by 2–9 cell layers of hypertrophied tissue. Hypertrophy lost in locules without fly/nematodes.
Morphology of nematodes. Parthenogenetic female medium size, C-shape; body narrows rapidly behind vulva; large oesophageal gland; conoid tail. Infective female larger than parthenogenetic, arcuate; cylindroid; short tail with broadly rounded tip; V 70–80%. Male medium size, straight to J-shape; oesophageal gland large; angular spicule; tail variable, slender or broad, with bluntly or broadly rounded tip; bursa 20–40%.
Morphology of dorsal shield. (WINC 004914, 004004, 004019, 003328, 003358, 003360, 003368-70). Shield a medial black sclerotised plate about 0.30–0.35 mm wide, formed by fusion of posterior portion TS 3, AS 1 and anterior portion of AS 2; usually with four, sometimes three and rarely five anterior projecting, stout, sclerotised teeth; raised sclerotised spicules on anterior dorsal margin of TS 1 and dorsally on TS 1 and 2; a transverse series of raised ridges comprising raised sclerotised spicules occur most prominently on AS 1– 4, becoming less prominent on AS 5–7. A similar shield, from TLG on E. cosmophylla , is shown in Fig. 68 View FIGURES 62–68 .
Possible relationships. Grouped in Clade 3; for discussion see under E. tereticornis TLG.
Galls have been collected from E. camaldulensis (river red gum) from both var. camaldulensis (found in southern QLD, VIC, and the Mount Lofty Ranges, Kangaroo Island, Eyre Peninsular and the South-East Region in SA) and var. obtusa (in the Flinders Ranges and northern desert areas of SA, and in the Kimberley and South West regions of WA).
From D2/D3 and COI sequences (Figs 78, 79), there appears to be some genetic drift between F. brittenae collected from E. camaldulensis in SA and WA ( Ye et al. 2007b). Based on D2/D3 and COI sequences, voucher specimens nos 205 and 55 (respectively, collected from Hall’s Creek in the north-east of WA and Goolwa in SA) are the same, but the position of the other samples (voucher specimens nos 62, 59, 6, 292, and 56; from Kellerberrin, WA, Marla, SA, Goolwa, SA, Brachina Gorge, SA, and Bunyeroo Gorge, SA, respectively) is unresolved and not well supported for D2/D3 (74%). Vouchers 55 and 6 were both collected from the one tree, albeit at different sampling times, suggesting that there may be an artifact in these analyses. The possibility of development of a species complex inducing terminal leaf bud galls on E. camaldulensis would not be surprising given its wide distribution, but against this is the finding that voucher specimens nos 205 and 55, from widely geographically separated areas, are genetically the same. More work is needed to show whether or not there is a species complex, or one species, of F. brittenae in these galls from E. camaldulensis .
(b) Flower bud galls
Fergusobia curriei (voucher specimens nos 3, 5, 206) associated with Fn. tillyardi Tonnoir 1937 View in CoL or an undescribed species of Fergusonina View in CoL ( Figs 4 View FIGURES 1–22 , 26 View FIGURES 23–43 , 47 View FIGURES 44–61 ). Clade 4 in Fig. 78.
Form of gall. Flower bud galls ( Currie 1937, Giblin-Davis et al. 2004a, Taylor et al. 2005). Galled buds of similar shape but much larger than uninfested buds; operculum lost in more mature galls. Galled tissue appears to be a proliferation of the disc and tissues, either from the base of the stigma or from the stamens.
Ovaries appear similar to those of uninfested buds. Anthers not seen, but remains of filaments are present.
Locules not membrane-bound. Unfortunately, no specific gall material was kept as vouchers for the samples sequenced here.
Morphology of nematodes. Parthenogenetic female open C-shape, medium size; oesophageal gland enormous; broad conoid tail with bluntly rounded tip. Infective female medium to large size, arcuate to C-shape with most curvature in posterior region; short tail with almost hemispherical tip; V 75–80%. Male medium to large size, arcuate to just J-shape; large oesophageal gland; tail tip bluntly rounded; angular spicule; bursa ca 15% body length.
Morphology of dorsal shield. While no specific fly voucher material was kept for the associated nematode samples sequenced here, all larvae examined from flower bud galls had similar shield morphology (K.A. Davies, unpubl. data). It was also similar to the ‘plates with teeth’ form commonly collected from TLG on host plants from the subgenus Symphyomyrtus . Here, the shield of fly larvae collected from Ambleside, near Adelaide, SA (WINC 003304) is described.
Fergusonina tillyardi . Dorsal shield heavily sclerotised, formed from a plate on AS 1 confluent with a short broad sclerotised plate on anterior margin of AS 2, and a short broad weakly sclerotised transverse band in the middle of AS 2. Seven to eight very long, sharp, forward projecting teeth arise from the posterior margin of AS 1. The middle teeth are broad, long and blade-like, and the outer teeth are narrower and more sharply tipped. There are raised spicules anterior to the shield on TS 3, lateral to it on AS 1 and lateral and posterior to it on AS 2.
Possible relationships. Clade 4, comprising different collections of Fergusobia from FBG on E. camaldulensis , was well supported (100%) overall, but the placement of the collections within the clade was poorly supported (58%). Genetic analyses of Fergusonina flies collected from flower bud galls on E. camaldulensis in Adelaide have shown that there are two species associated with these galls (S. Scheffer, unpub. data). However, no material was available for comparisons of gall morphology, fly larval shields or of associated nematodes. An intensive sampling of these gall forms from the South Australian region is planned to further examine this gall form it and the associated flies and nematodes.
Given the morphological similarity of the fly larval shield types and of the nematodes, and coming from the one host, it is not surprising that Clades 3 and 4 of nematodes forming TLG and FBG from E. camaldulensis inferred from D2/D3 sequences are genetically close (Fig. 78). This could be a duplication event for these lineages, which in turn suggests that the fly/nematode mutualism evolved with the host plant, either by coevolution or association by descent (common ancestor) .
(c) ‘Stem’ shoot bud galls
Fergusobia Morphospecies 10 (voucher specimens nos 54, 311); associated with an undescribed species of Fergusonina
( Figs 16 View FIGURES 1–22 , 38 View FIGURES 23–43 , 58 View FIGURES 44–61 ). Clade 15 in Fig. 78.
Form of gall. ‘Stem’ galls ( Taylor et al. 2005) are multilocular axilliary vegetative bud galls. Rather nodular, warty multilocular galls with three to five locules, lacking a specific shape. Given their axilliary origin, and because their growth causes dehiscence of the leaf, they frequently appear to be developing on stems. Most found during the Australian spring ( Head 2008), but occasional galls appear in autumn.
Morphology of nematodes. Parthenogenetic female medium size; shape arcuate to almost straight; distinct conoid tail with broadly rounded tip. Infective female large; arcuate, cylindroid; short broad tail with sub-truncate or hemispherical tip; V 75–85%. Male medium size; arcuate, C to J-shape; tail tip bluntly rounded; spicule angular, not strongly sclerotised; bursa 45–80%.
Morphology of dorsal shield. (WINC 003304, 003314-5, 003317). Shield comprises 9 transverse ‘bars’ of raised sclerotised spicules. Widest and with most spicules on TS 2, TS 3 and AS 1. Figure 63 View FIGURES 62–68 illustrates this form of shield, but from larvae collected from a FLG on E. sp.
Possible relationships. Clade 15, inferred from sequences of D2/D3, comprises two collections of these nematodes. The clade was placed close to the previous clade of pea gallers (clade 14), but with poor support (Fig. 78). Analyses of sequences from COI (Fig. 79) place the nematodes close to those from M. linariifolia (voucher specimen no 412) but also with poor support. Nematode morphology from all three clades was similar, in that all have straight or arcuate parthenogenetic females with extensile uteri. Larval morphology of associated flies from the three clades was also similar, representing varying degrees of development of the ‘bar’ form of the shield (from no shield to a few spicules to definite bars of spicules; or vice versa, depending on whether the lack of shield is seen as the primitive or derived condition). Sequencing of a larger sample of nematodes from similar gall forms, and associated with flies having similar shield morphology, is needed to clarify the relationships of these clades.
Given the wide distribution of the host E. camaldulensis , these mutualisms may have had a common fly/ nematode ancestor, and/or host switching may have occurred.
The shoot bud gall is the third gall form collected from E. camaldulensis (see above). The morphology of the fly larvae (shield made of bars of sclerotised spicules) and nematodes (straight or arcuate in shape) from the stem galls contrasts with that of the flies (shields comprising medial plates with teeth) and nematodes (Cshaped parthenogenetic females and J-shaped males) from TLG and FBG on the same host. This supports molecular data (Fig. 78) showing that the fly/nematode mutualism from the shoot bud galls on E. camaldulensis developed from a different lineage to that of the TLG and FBG.
Section Maidenaria
Fergusobia Morphospecies 11 (voucher specimens nos 347, 273); associated with an undescribed species of Fergusonina ( Figs 22 View FIGURES 1–22 , 43 View FIGURES 23–43 , 61 View FIGURES 44–61 ). Unplaced with D2/D3.
Form of gall. ‘Leafy’ shoot bud galls ( Fig. 14 View FIGURES 1–22 in Taylor et al. 2005). Galls consist of coalesced gall tissue, the locules held in a single plane by expanding leaf tissue, so that leaf and meristematic stem tissue covers an irregularly shaped gall, usually with leaf tissue growing beyond it.
Morphology of nematodes. Parthenogenetic female small, open C-shape; large oesophageal gland; body narrows rapidly behind vulva; short conoid tail, with bluntly or broadly rounded tail tip. Infective female medium size, C to J-shape; body width greatest at vulva; body strongly curved behind vulva; broadly rounded tail tip. Male medium size, J-shape; oesophageal glands medium to large; angular spicules; short bursa.
Morphology of dorsal shield. (WINC 003119-21) ( Fig. 65 View FIGURES 62–68 ). Shield comprises 2 patches of heavily sclerotised cuticle, without surrounding raised spicules. The first is a large sclerotised area on the posterior margin of TS 3 confluent with a short broad area on anterior margin of AS 1. Second is a narrow sclerotised patch on posterior margin of AS 1 confluent with a short sclerotised patch on anterior margin of AS 2.
Possible relationships. Eucalyptus viminalis is a common tree in the wet forests of NSW, VIC and TAS. In SA, it is restricted to higher parts of the Mount Lofty Range.
Interestingly, while nematodes from galls collected in NSW and SA appear to be different species from D2/D3 analysis (voucher specimens nos 347 and 273, Fig. 78), whose positions in the inferred tree were not supported, molecular analysis of the associated flies showed that they were the same species (S. Scheffer, unpub. data). It is unclear if the apparent differences in sequences from the nematodes are a) artifact or b) indicate that there has been genetic drift in the nematodes, possibly reflecting geographic distance or multiple foundresses. Further work on the fly/nematode mutualism is needed.
The Fergusobia / Fergusonina View in CoL galls collected from E. viminalis View in CoL had a similar form to that of galls from E. stuartiana View in CoL now E. bridgesiana View in CoL pictured by Currie (1937), and the morphology of the respective associated fly larvae is also similar (see Fig. 11 View FIGURES 1–22 in Currie 1937). The distributions of both host species overlapped, and both belong to Section Maidenaria, but to differing Series (respectively, Viminales and Bridgesiana View in CoL ). Thus, Fergusobia Morphospecies 11 may be close to the type species for the genus, F. tumefaciens . While the sketches in Currie (1937) do suggest similarity in body shape of the nematodes from E. viminalis View in CoL and F. tumefaciens , recollection of the latter is needed to assess morphological similarity, and molecular identity.
‘Leafy’ leaf bud galls also occur on E. planchoniana View in CoL , a host species with a disjunct distribution from that of E. viminalis View in CoL , and lacking close genetic similarity with it (from subgenus Eucalyptus View in CoL and Symphyomyrtus respectively). This seems an example of similarity of gall form reflecting the particular meristematic tissue galled.
Section Adnataria
(a) Flower bud galls
Fergusobia voucher specimen no 1; associated with an undescribed species of Fergusonina ( Figs 5 View FIGURES 1–22 , 27 View FIGURES 23–43 , 48 View FIGURES 44–61 ). Clade 5 in
Fig. 78.
Form of gall. Flower bud galls ( Currie 1937, Giblin-Davis et al. 2004a, Taylor et al. 2005), club-shaped, larger than uninfested flower buds. Locules appear to originate in a proliferation of anther cells, and each is attached to floret wall by an anther filament. Filaments in infested buds have 2–3x the diameter of those in uninfested buds. Locules membrane-bound. Operculum not released.
Morphology of nematodes. Parthenogenetic female open C-shape, medium size; oesophageal gland large; short conoid tail, tip broadly rounded. Male medium to large size, J-shape; oesophageal gland medium; angular spicule; bursa ca 20% body length.
Morphology of dorsal shield. (WINC 004878). Shield comprises a heavily sclerotised, broad plate on posterior margin of TS 3, confluent with one on AS 1, and a shorter plate on anterior margin of AS 2. Two short, forward projecting, blunt teeth arise from the posterior margin of AS 1.
Possible relationships. Clade 5 comprised these nematodes from E. microcarpa and also those forming PG from E. microcarpa , and FBG from E. fibrosa . Analyses of sequences from D2/D3 suggested that the nematodes were grouped, but with no support. With COI sequences, grouping of nematodes from E. fibrosa and from C. tessellaris was strongly supported (Fig. 79). The latter does not make biological sense, as the shield form of the associated flies differs, the respective nematodes have different morphologies, and the gall forms differ; and while the host plants have overlapping distributions in Queensland, genetically they are widely disparate coming from different genera.
The distributions of E. microcarpa and E. fibrosa overlap; and both are classified in the Section Adnataria but from two series (i.e., they have some genetic similarity). The associated fly larvae had similar shield forms, nematode morphology was similar, and the gall forms on the host plants were similar. This suggests that the flies and nematodes respectively come from the same lineages, and that coevolution occurred with the host plants, or there was a host switch.
(b) Flat leaf galls
Fergusobia Morphospecies 12 (voucher specimen no 67); associated with an undescribed species of Fergusonina . Clade
13 in Fig. 78.
Form of gall. Flat leaf galls, formed when flies apparently oviposit into the surface of a newly expanding leaf blade ( Giblin-Davis et al. 2004a, Taylor et al. 2005). Galled leaves have rows of oviposition scars on the leaf surface, each with a gall locule developing beneath it. They consist of a single layer of many locules in a more or less mature leaf. All or part of the whole leaf blade is thickened by 5–10 mm, with little expansion of the leaf area.
Morphology of nematodes. Parthenogenetic female small size; open C-shape; large oesophageal glands; short conoid tail with broadly rounded tip. Infective female small to medium size; straight to arcuate or open C-shape; cylindroid; short broad tail; V 80–90%. Male small to large; almost straight to J-shape; medium to large oesophageal glands; short tails varying in width; weak angular spicules; bursa 25–50% body length.
Morphology of dorsal shield. (WINC 004853, 004861-3, 004866-7). Shield comprises two patches of heavily sclerotised cuticle. First is a patch at the posterior margin of TS 3, confluent with a patch at the anterior margin of AS 1, with areas of sparse raised sclerotised spicules anterior and posterior to it. Second occurs at the posterior margin of AS 2 confluent with a patch on the anterior margin of AS 3. The first patch is larger than the second. Figure 64 View FIGURES 62–68 shows a shield with similar form from larvae from a PG on E. porosa .
Possible relationships. Grouped in Clade 13; see discussion under E. eugenioides .
The distribution of E. microcarpa overlaps those of E. eugenioides , E. siderophloia , E. leucoxylon and E. porosa . Eucalyptus porosa and E. microcarpa are closely related, and the nematodes collected from them may have cospeciated with their hosts.
(c) Unilocular axial pea galls
Fergusobia voucher specimen no 70; associated with an undescribed species of Fergusonina . Clade 5 in Fig. 78.
Form of gall. Unilocular axial ‘pea’ gall; spheroid, stalked.
Morphology of nematodes. Parthenogenetic female medium size; open C-shape; large oesophageal gland; short conoid tail with broadly rounded tip. Male small; J-shape; medium oesophageal glands; short broad tails with broadly rounded tip; angular spicules; bursa ca 30% body length.
Morphology of dorsal shield. (WINC 004869). Dorsal shield comprising confluent plates of plain, heavily sclerotised cuticle. First, a heavily sclerotised plate on posterior margin of TS 3, confluent with a broader one on AS 1; confluent with a shorter plate on posterior margin of AS 1; confluent with a plate on anterior margin of AS 2. Patches of raised heavily sclerotised spicules occur on central areas of AS 2–5.
Possible relationships. Grouped in Clade 5; see discussion under E. microcarpa FBG.
(a) Flat leaf galls
Fergusobia voucher specimen no 66; associated with an unknown species of Fergusonina . Clade 13 in Fig. 78.
Form of gall. Flat leaf galls ( Giblin-Davis et al. 2004a, Taylor et al. 2005). As above, for E. microcarpa .
Morphology of nematodes. Parthenogenetic female small to medium size; open C-shape; large oesophageal gland; short conoid tail with bluntly rounded tip. Infective female small size; straight to arcuate or open C-shape; cylindroid; short broad tail; V ca 85%. Male small size, arcuate to J-shape; large oesophageal gland; short tail; weak angular spicule; bursa 15–30% body length.
Morphology of dorsal shield. (WINC 004416, WINC 004270). Shield comprises patches of sclerotised cuticle; the first a small area on the posterior margin of TS 3, confluent with a smaller, heavily sclerotised patch on the anterior margin of AS 1; second a small sclerotised area on the posterior margin of AS 1 confluent with a smaller sclerotised area on the anterior margin of AS 2. Raised sclerotised spicules occur around and between the patches of sclerotised cuticle. Fig. 66 View FIGURES 62–68 shows a shield with similar form from FLG on E. odorata .
Possible relationships. Grouped in Clade 13; see discussion under E. eugenioides and E. microcarpa .
(b) Unilocular ‘pea’ galls on leaflets
Fergusobia Morphospecies 13 (voucher specimen no 69); associated with an unknown species of Fergusonina . Clade 13 in Fig. 78.
Form of gall. Unilocular ‘pea’ galls on developing leaflets within buds. Similar to those described from E.
gomphocephala.
Morphology of nematodes. Parthenogenetic female small to medium size; open C to C-shape; large oesophageal gland; small conoid tail with rounded tip. Infective female small to medium size; straight to arcuate; cylindroid; tail short with hemispherical tip; V ca 85%. Male small to medium; almost straight to arcuate; short arcuate tail with bluntly rounded tip; angular spicules; bursa 15–33% body length.
Morphology of dorsal shield. From E. porosa (WINC 003007). Shield comprising a heavily sclerotised broad plate on posterior margin of TS 3, confluent with one on AS 1, and on anterior margin of AS 2. Four anteriorly projecting, sharp teeth arise from posterior margin of AS 1.
Possible relationships. Grouped in Clade 13; see discussion under E. eugenioides and E. microcarpa .
Series Siderophloiae
Fergusobia Morphospecies 14 (voucher specimen no 330); associated with an undescribed species of Fergusonina . Clade 5 in Fig. 78.
Form of gall. Flower bud galls; club-shaped, larger than uninfested flower buds. Have not been histologically examined.
Morphology of nematodes. Parthenogenetic female C-shape, medium size, enormous oesophageal gland;
narrow conoid tail, bluntly rounded tip. Infective female medium to large, arcuate to J-shape; vulva 73–80%;
short tail with almost hemispherical tip. Male medium to large size, arcuate to just J-shape; large oesophageal gland, tail tip bluntly rounded; angular spicule; bursa 75–80% body length.
Morphology of dorsal shield. (WINC 004201). Shield extends over three segments, comprises three fused plates. First, a broad sclerotised plate on TS 3, taking up most of its length; second a plate comprising the length of AS 1, with two sub-medial forward projecting hooked teeth and posterior edge developed into a backward projecting ridge; confluent with a third which is a sclerotised plate on the anterior of AS 2. Sclerotised raised spicules are absent.
Possible relationships. Grouped in Clade 5; see discussion under E. microcarpa .
Fergusobia voucher specimens nos 25, 26, 27, 28; associated with an undescribed species of Fergusonina . Clade 13 in Fig. 78.
Form of gall. Flat leaf galls ( Giblin-Davis et al. 2004a). Composed of a series of locules along the leaf blade that alternate between those with typical hypertrophied cell layers and others without the hypertrophy and cells like those of the matrix, i.e., the leaf only thickens in the area of the locules. Locules formed in parenchymatous tissue, with no differentiation of cells into palisade and spongy parenchyma. Vascular tissues and oil glands scattered in gall matrix.
Morphology of nematodes. Parthenogenetic female medium size; open C-shape; large oesophageal glands; short, relatively slender conoid tail with bluntly rounded tip. Male medium size, J-shape; medium oesophageal gland; short tail; weak angular spicules; bursa ca 30% body length.
Morphology of dorsal shield. (WINC 003410) ( Fig. 67 View FIGURES 62–68 ). Shield comprises a sclerotised cuticular patch on the posterior margin of TS 3, confluent with a broad sclerotised patch on anterior margin of AS 1. There is a row of raised sclerotised spicules behind this. A second sclerotised patch occurs on the anterior margin of AS
2, with lateral areas of sparse scattered spicules. Also an area of sparse scattered spicules on anterior margin of
AS 3.
Possible relationships. Grouped in Clade 13; see discussion under E. eugenioides .
Section Heterophloiae
Fergusobia Morphospecies 15 (voucher specimen no 65); associated with an undescribed species of Fergusonina ( Figs 7 View FIGURES 1–22 , 29 View FIGURES 23–43 , 50 View FIGURES 44–61 ). Unplaced in Fig. 78.
Form of gall. Stigma gall ( Taylor et al. 2005). Inconspicuous, with one to three locules. Gall is indicated by a swollen flower stigma, after the shedding of the operculum and dehiscence of stamens. An apparently unique gall form.
Morphology of nematodes. Parthenogenetic female small; arcuate; with short, broadly conoid tail. Infective female open C-shape; hemispherical tail tip. Male J-shape; spicules angular; short peloderan bursa.
Morphology of dorsal shield. (WINC 003028). Shield comprises 3 confluent plates; the first a heavily sclerotised plate on the posterior margin of TS 3 confluent with a broad heavily sclerotised plate on AS 1 and a short, broad, heavily sclerotised plate on the anterior margin of AS 2. Anterior to the plate on TS 3 there is an area of sparse, raised sclerotised spicules. A row of 8–10 short, sclerotised, anterior projecting, sharp teeth arises from the posterior margin of AS 1. A second row of much smaller teeth arises from the anterior margin of AS 2.
Possible relationships. Fly larval shield morphology from these galls is similar to that from nematodes forming TLG and FBG on other known host eucalypts in Subgenus Symphyomyrtus , Section Adnataria. Morphologically, the nematodes are also similar to those from hosts within the Adnataria.
The data suggest there are respective common lineages for the flies and nematodes, with differing gall forms depending on the types of meristem infested. It is not clear if this is an example of coevolution or host-switching.
Series Melliodorae
(a) Leaf ‘pea’ galls
Fergusobia Morphospecies 16 (voucher specimens nos 68, 314); from E. leucoxylon associated with an undescribed species of Fergusonina . Clade 7 in Fig. 78.
Form of gall. Small, pea-like galls on young leaves. Similar to unilocular (discrete) leaf galls, but differ in that the galls have 1 to 3 hemispherical locules.
Morphology of nematodes. Parthenogenetic female small size, arcuate, open C-shape, oesophageal glands large; body narrows gradually behind vulva; short broad conoid tail, with broadly rounded tip. Infective female small, open C-shape; maximum body width at vulva; short broad tail; V 70–85%. Male small size, Jshape; oesophageal gland enormous; spicule weakly sclerotised, angular; short tail with broad tip; bursa ca 10% body length.
Morphology of dorsal shield. (WINC 003162) No larval material. Pupal shield comprises a heavily sclerotised broad plate on posterior margin of TS 3, confluent with one on AS 1, and on anterior margin of AS 2. A raised ridge arises from posterior margin of AS 1, bearing 3 or 4 strongly curved, anterior projecting, sharp teeth.
Possible relationships. Grouped in Clade 7; see discussion under E. gomphocephala .
(b) Small bud galls
Fergusobia Morphospecies 17 (voucher specimen no 71); associated with an undescribed species of Fergusonina . Clade 8 in Fig. 78.
Form of gall. Small bud galls with 1 to 5 locules on leaf bud ( Fig. 85 View FIGURES 80–88 ).
Morphology of nematodes. Parthenogenetic female small, open C-shape; large oesophageal gland; tail conoid with a broadly rounded tip. Male small, barely J-shape; tail tip broadly rounded; angular (not heavily sclerotised) spicules; bursa ca 20% body length.
Morphology of dorsal shield. (WINC 003156) Shield comprises 2 patches of heavily sclerotised cuticle.
First is a large sclerotised depression on the posterior margin of TS 3 confluent with a short broad depression on anterior margin of AS 1. Second is a short, broad sclerotised depression on posterior margin of AS 1 confluent with a short broad sclerotised depression on anterior margin of AS 2. A similar shield, from a unilocular bud gall on E. viminalis , is shown in Fig. 65 View FIGURES 62–68 .
Possible relationships. Grouped in Clade 8; see discussion under E. planchoniana .
(c) Flat leaf galls
F. fisheri Davies & Lloyd 1996 (voucher specimen no 8) from E. leucoxylon View in CoL ; associated with an undescribed species of Fergusonina View in CoL ( Figs 14 View FIGURES 1–22 , 36 View FIGURES 23–43 , 56 View FIGURES 44–61 ). Clade 13 in Fig. 78.
Form of gall. Flat leaf galls ( Giblin-Davis et al. 2004a, Taylor et al. 2005). As for FLG from E. microcarpa View in CoL ; see above.
Morphology of nematodes. Parthenogenetic female small size; open C-shape; large oesophageal gland;
short conoid tail with bluntly rounded tip. Infective female small size; arcuate to open C-shape; cylindroid;
short broad tail with tip almost hemispherical; V 75–90%. Male small, variable shape; medium to large oesophageal gland; short tail with bluntly rounded tip; angular spicule; bursa ca 20% body length.
Morphology of dorsal shield. (WINC 004895, 004898, 003384, 004893, 003400) Shield comprises two patches of heavily sclerotised cuticle. First, a small sclerotised patch on the posterior margin of TS 3, confluent with a larger patch on anterior margin of AS 1, surrounded by a few sparse raised sclerotised spicules. Second, a smaller sclerotised patch on anterior margin of AS 2, with a few surrounding sclerotised spicules.
Possible relationships. Grouped in Clade 13; see discussion under E. eugenioides .
d) Axial bud galls
Fergusobia voucher specimen no 741; associated with an undescribed species of Fergusonina . Clade 7 in Fig. 79.
Form of gall. Small axial bud galls with 2 to 5 locules.
Morphology of nematodes. Parthenogenetic female small, C-shape; large oesophageal gland; tail conoid with a narrowly rounded tip. Male small, almost straight shape; tail tip rounded; spicules angular, not heavily sclerotised; bursa ca 20–30% body length.
Morphology of dorsal shield. (WINC 063701) Shield comprising a heavily sclerotised broad plate on posterior margin of TS 3, confluent with one on AS 1, and on anterior margin of AS 2. A raised ridge arises from posterior margin of AS 1, bearing 3 or 4 strongly curved, anteriorly projecting, sharp teeth.
Possible relationships. Voucher specimen no 741 did not sequence for D2/D3. With COI, a group (90% support) of these nematodes and other Fergusobia from E. leucoxylon (voucher specimen no 71), E. cladocalyx (voucher specimen no 740) and E. sp. (voucher specimen no 32) was inferred. While the fly larvae associated with voucher specimen no 71 had shields of the ‘two patches’ form, the other fly larvae associated with this clade all had shields of the ‘plates with teeth’ form. It is possible that one lineage of nematodes has become associated with two lineages of flies.
The three different forms of shield on the fly larvae collected from various leaf bud galls on E. leucoxylon is evidence for three different Fergusobia / Fergusonina associations on this host. Morphologically, the nematodes forming LBG cannot be separated, and the 3 rd stage Fergusonina fly larvae are required for initial identification of the particular nematode/fly association. This is further evidence that for the Fergusobia / Fergusonina mutualism, gall form is determined by the particular meristematic tissue targeted by the fly.
| L |
Nationaal Herbarium Nederland, Leiden University branch |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Corymbia
| Davies, Kerrie A., Ye, Weimin, Giblin-Davis, Robin M., Taylor, Gary S., Scheffer, Sonja & Thomas, W. Kelley 2010 |
Fergusobia brittenae
| Davies 2010 |
Fergusobia ptychocarpae
| Davies 2008 |
Fn. giblindavisi Taylor 2008
| Taylor & Davies 2008 |
F. fisheri
| Davies & Lloyd 1996 |
Fergusobia curriei
| Fisher and Nickle 1968 |
F. tumefaciens
| Filipjev and Schuurmans Stekhoven 1941 |
F. tumefaciens
| Filipjev and Schuurmans Stekhoven 1941 |
Fn. newmani
| Tonnoir 1937 |
Fn. lockharti
| Tonnoir 1937 |
Fn. tillyardi
| Tonnoir 1937 |