Tyrannosaurus rex, Osborn, 1905
|
publication ID |
https://doi.org/10.1080/0891296021000050755 |
|
DOI |
https://doi.org/10.5281/zenodo.3811873 |
|
persistent identifier |
https://treatment.plazi.org/id/FC378752-FFEF-1244-FA84-1E940CBDF5F1 |
|
treatment provided by |
Jeremy |
|
scientific name |
Tyrannosaurus rex |
| status |
|
Existing measurements of bite force were compiled from the literature ( Table I View TABLE I ) and analyzed in relation to the mean body masses of a variety of predatory species. The measurements of bite force were obtained in a variety of ways, ranging from electrically mediated muscle stimulation to voluntary bites of trained animals and estimates of force generation based on the cross-sectional areas of jaw adductor muscles. To maximize the amount of data available for this analysis, results obtained in all these different ways were accepted as being of equally validity. In fact, however, it is unclear whether or not actual maximum bite forces are obtained by any of the methods, particularly in the case of measurements obtained from trained animals and estimates inferred from morphological analyses. This analysis was restricted to amniotes that are predominantly predators. Herbivores and gramnivores were excluded due to the obvious differences in morphology and function of their teeth and jaws, called for by their choice of food. Consequently, the species available for analysis are somewhat limited in variety, although they include crocodylians, mammals, chelonians, and squamates. Fully aquatic predators, such as sharks and toothed whales, were excluded due to the effects of buoyancy on body mass. In a gravitationally neutral environment, body mass may increase with greater positive allometry than is likely to be found among terrestrial predators. As a consequence, the inclusion of sharks or other aquatic predators would obscure any relationship that may exist between body mass and bite force in land animals. Actual body masses of test subjects were retained in analyses where available, while species mean body masses were utilized in the absence of direct measures from bite force subjects (see Table I View TABLE I ).
Published data were searched for references to the largest known prey items taken by each terrestrial predator, to establish the upper limits of their choice of prey. Unfortunately, prey are typically referred to in generalized, even colloquial terms in the ecological literature. For example, "rabbits" are cited as prey for a variety of predators, without listing the species taken. Hence, the species-level identification of prey is often impossible. In these cases, prey items were defined as clades, at levels implied by their informal identification, and the mean body mass of species included in each clade was used to represent prey body mass. Where prey items are precisely identified, the mean body mass of the species was used in the analysis.
Bite force and the body mass of the largest known prey taken by each predator were plotted separately against the body masses of predatory species. The observed correlations were analyzed using least squares regression. Estimates of analogous relationships between extinct predators and their prey can be made using these analyses. However, in the case of extraordinarily large animals, such as the theropod dinosaur Tyrannosaurs rex and its prey, this requires extrapolation beyond the range of data provided by extant taxa. Since such extrapolation is inherently more speculative than inferences based on overlapping size ranges, 95% confidence intervals for slopes and means were calculated using the commercially available software StatView from SAS. Predictions that have been transformed back to natural units from logarithms can be problematic as a result of what has been called logarithmic transformation bias. This results in a tendency to underestimate the value of the dependent variable ( Smith, 1993). In this study, the Ratio Estimator (RE) correction factor described by Snowdon (1991) was used to generate final, corrected values for the prediction of maximum bite force (see Table III). The RE correction factor is the ratio of the mean of observed values of the dependent variable, divided by the mean of values predicted by transformation back from logarithms that define an exponential relationship. Effectively, this correction is based on the average difference between actual values of the dependent variable and corresponding values predicted by the regression equation.
RESULTS
Bite Force /Body Mass
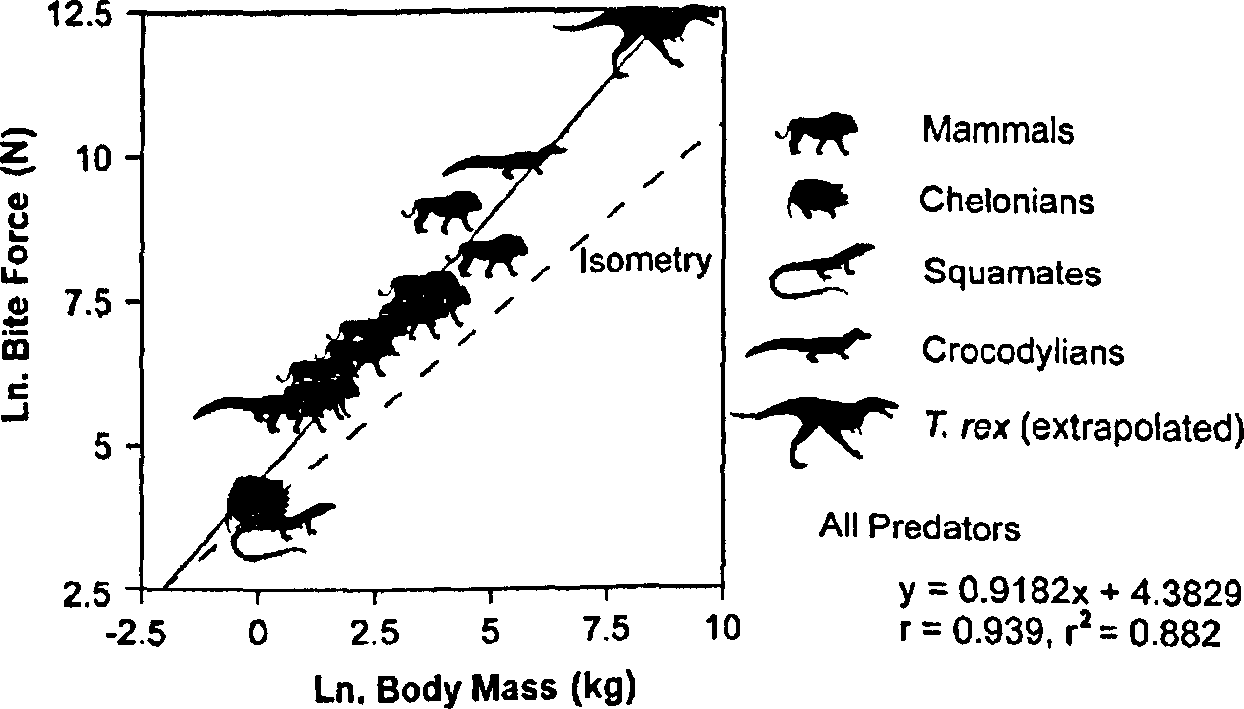
Bite force is highly correlated with the predator's body mass (r = 0.939; r2 = 0.882) among those extant predators for which data are available ( Fig. 4 View FIGURE 4 , Table II). Restriction of the sample to more narrowly defined groups of phylogenetically related taxa yields similar correlations. For example, among mammals, r = 0.930 ( r 2 = 0.864). Analysis of the bite force data available from crocodylians, the closest living relatives of T. rex , is less useful. Only two species have been studied, and the values determined for one species (Caiman crocodilus) vary substantially among workers ( Cleuren et al., 1995).
Group Y-intercept 95% Upper and lower Slope 95% Upper and lower r r 2 p-value
All predators 4.383 4.907 0.918 1.103 0.939 0.882 <0.0001
3.859 0.733 Crocodylians 5.379 - 0.785 - 1.0 1.0 Mammals 5.031 5.694 0.725 0.942 0.930 0.864 <0.0001
4.368 0.508
Extrapolation from the relationship derived by regression based on these observations of extant taxa to 5371kg, the body mass of T. rex , yields an estimated maximum bite force of 235,000 N ( Table HI). The body mass used here is the more conservative of estimates determined by Farlow et al. (1995). Alternatively, if crocodylians alone are used to derive an estimate for maximum bite force in T. rex , the estimate would be only 183,000N. Use of the regression based on mammals yields a much lower figure, 88,000 N ( Table III).
Prey/Predator Body Mass
The body masses of predators and their largest prey ( Fig. 5 View FIGURE 5 , Table IV) are less highly correlated ( r = 0.795; r2 = 0.631). Coincidentally, this correlation is the same as that reported by Vezina (1985) for the relationship between predator and prey body masses in general. So, the relationship of maximum prey body mass to predator body mass is as strong as that established for the more general correlation between prey and predator body masses. The precision of the correlation observed here is probably limited in part by uncertainty associated with the prey body mass data, noted above.
Partitioning of the data by feeding strategy, between animals that hunt alone or in packs for example, makes it possible in some cases to evaluate possible predatory strategies in extinct taxa. In this analysis, T. rex was paired with its most commonly inferred prey, Triceratops horridus , to assess the possibility of a predator-prey relationship between them. This particular paleoecological relationship does fall within the 95% confidence interval for the range of values among solitary predators. In fact the relationship between these two taxa falls within the range of those found among extant taxa, regardless of predatory strategy.
DISCUSSION
A strong relationship between bite force and body mass across a wide variety of taxa is clear, given the results presented above. Given that biting in crocodylians closely mirrors our expectations for the feeding of theropod dinosaurs in its mechanical function ( Farlow, 1976), it might be expected that these would prove to be the most useful modem analogues. However, crocodylians do not typically kill large prey by biting. They subdue their prey by means of various subsequent actions, including shaking and drowning (personal observations). The possibility that T. rex used similar tactics could be investigated through study of cervical biomechanics and other skeletal features. The main problem at present is the relative paucity of data that has so far been obtained from crocodylians. The available data simply do not provide an adequate basis on which to derive estimates applicable to dinosaurs.
Analysis of muscle physiology can be used to constrain and evaluate the results obtained here. How much muscle mass would be required to exert the 235,000 N bite force inferred for T. rex in this study? Most vertebrate muscle that has been tested exhibits a maximum contractile force of approximately 30 N/cm2, although values of up to 38 N/cm2 ( Jayes and Alexander, 1982) have been reported. Using the simplified calculations of Greaves (1995), it can be shown that muscle with approximately 6,300-8,000 cm2 of physiological cross-sectional area would be required to produce a bite force of 235,000N. The actual value, within this range of possibilities, depends on the contractile properties of specific muscle fibers and their mechanical advantage. The cross-sectional areas of jaw adductor muscles were not determined in this study, but these figures do not seem to be out of the question for T. rex , particularly if some muscles had an architecture other than strictly parallel fibers.
Using muscle reconstructions that assumed parallel fibers, Rayfield et al. (2001) recently determined that the bite force of the markedly more gracile Allosaurus fragilis would have been 18,747 N, which is well below the estimate arrived at here for T. rex . This makes lower estimates possibly more intriguing. Use of the crocodylian data alone leads to a predicted bite force of about 183,000 N, which would require jaw adductors with less than 6/300 cm2 of physiological cross-sectional area. However, uncertainties associated with the crocodylian data remain an important consideration. Use of the mammalian data alone may be even more problematic, for a variety of reasons. To complete the argument, we note that a predatory mammal with a body mass in the range of T. rex would be expected to develop a bite force of 88,000 N, which is less than half the force inferred from either the crocodylian data or the data for all taxa. In mammals, however, the maximum bite force occurs at the molars. Mammalian molars are highly specialized for functions unlike those of theropod teeth, so a force based on extrapolation from mammal data is unlikely to have much meaning for T. rex .
The estimate of bite force in the theropod dinosaur T. rex calculated on the basis of data from all taxa included in this study (crocodylians, mammals, and squamates) is the most rigorous prediction possible at this time, notwithstanding its relatively high value, at 235,000 N. The high correlation of observed bite force with body mass among living predators ( r = 0.939) suggests that the relationship between these variables is quite strong and that the actual bite force fell within the range estimated here.
The intriguing bite marks made by T. rex , described by Erickson and Olson (1996) and subsequently analyzed by Erickson et al. (1996), must be taken into account in this analysis of maximum bite force. Erickson et al. (1996) calculated the force required to make a particular bite mark on the pelvis of a Triceratops at approximately 6,400 N for a single, mid-maxillary tooth. If we assume that the other teeth in the maxilla could be similarly engaged, although this is not likely to have been the case for their specimen, due to its mode of engagement with the prey, the force exerted by this tooth may represent an average force per tooth. In this case, the total force exerted in a bilateral bite of this sort can be calculated as:
6,400 N x 12 maxillary teeth x 2 maxillary tooth rows
= 153,600 N
This estimate is somewhat lower than those arrived at in this work, but it is of the same general magnitude. In any case, Erickson and Olson (1996) make a convincing case that the bite marks they observed were produced post-mortem, so the bite is unlikely to have matched the maximal force of which the predator was capable.
The maximum bite forces calculated here represent the total force that can be exerted in a bite. In a feeding situation where all jaw muscles are maximally activated, these forces would be divided among the teeth engaged. So, given twelve teeth in each maxilla, a bite of 235,000 N would represent an average force of 9,800 N at each tooth. In practice, these values are expected to be higher near the jaw joint and lower rostrally, given a simple secondorder lever model. This force is approximately 50% greater than the 6,400 N force determined by Erickson et al. (1996) for a mid-maxillary tooth (13,400 N for a posterior tooth) from T. rex bite marks on a Triceratops pelvis that was bitten post-mortem. A comparable analysis based on the lower maximal bite force derived from the crocodylian data alone suggest a lower average force per tooth of 7,600 N, which is about 20% higher than the force implied by bite marks made during the non-lethal, post-mortem phase of predation. Use of the mammalian regression leads to an estimated average force of only 3,670 N per tooth, a maximum value that is effectively falsified by the results of Erickson et al. (1996). This confirms the inadvisability of using the mammalian data alone to arrive at an estimate applicable to T. rex . Of course, the teeth of T. rex varied greatly in size and to a lesser extent in shape, along the jaw, so the distribution of bite force among them must have been complex. The larger caniniform teeth may reasonably be expected to have borne more force than the most posterior teeth, despite the potential to exert higher forces nearer the jaw joint.
The relatively high bite forces inferred in this study do not support the inference of direct predation by T. rex on large, adult ornithischian dinosaurs unless it is supposed that about 235,000 N was sufficient to kill the prey. Some of these animals, such as T. horridus , may have topped 5,000 kg in body mass ( Norman, 1985). High bite forces are characteristic of some of the most dedicated mammalian predators, but this feature alone seems to have little bearing on the distinction between predatory and scavenging habits. The spotted hyena, Crocuta crocuta , which is admittedly both a competent predator and a scavenger, exerts comparatively high bite forces in crushing bone ( Binder and van Valkenburgh, 2000). Interestingly, Farlow et al. (1991) suggested that large theropod teeth may have been used in crushing bone, based on their study of tooth strength and the frequency of broken and worn theropod teeth ( Carpenter, 1979; Farlow and Brinkman, 1987; Molnar and Farlow, 1990). Earlier, Paul (1988) had noted that the sheer heights of theropods would have necessitated attack from above, leading to relatively frequent encounters between teeth and bone. Although Farlow et al. (1991) observed that tooth-marked bones from the Mesozoic are rare, the recent description of a T. rex coprolite containing large amounts of broken bone ( Chin et al., 1998) further supports the hypothesis of bone crushing by T. rex . It may require more direct evidence than is so far available to determine whether such bones were crushed in the act of subduing prey or as a result of scavenging activities. Body size relationships and the strategies employed by predators may be more indicative of feeding behavior than the forms of teeth and jaws, as predator body mass necessarily limits the maximum size of prey that can be taken. While most predators take prey of their own size or smaller, social predators can take much larger prey. For example, gray wolves ( Canis lupus ) hunting in packs have occasionally been known to take moose (Alces alces), while large solitary predators such as tigers ( Panthera tigris) take proportionally smaller prey, such as the guar (Bos gaurus) ( Nowak, 1991). This relationship is in part driven by the predator's vulnerability to defensive strategies adopted by the prey, which have greater consequences with increasing prey size. Consequently, the prey available to a predator depend on body mass relationships and the behaviors of both predator and prey, with bite force playing a secondary role in conjunction with these other variables.
The relationship between predator and prey body masses documented in this study ( Fig. 5 View FIGURE 5 ) indicates a strong correlation between these variables that is independent of predatory behavior. Given analogous relationships among Late Cretaceous dinosaurs, an adult T. horridus falls well within the likely range of prey for T. rex , whether T. rex was a solitary or a social predator. Indeed, these data suggest that a social group of large theropod dinosaurs, such as Allosaurus , would have been capable of taking prey much larger than themselves, possibly including adult sauropods. As Farlow (1976) noted, the concentration of a mammoth potential food source in the form of sauropod herds would undoubtedly have attracted multiple theropods to a relatively confined geographic area, increasing the potential for emergence of social hunting in one form or another. Social hunting in this context does not have to imply the derived social organization and complex strategies seen in mammalian pack-hunters such as lions or wolves, It may have been much simpler, as seen in the behavior of opportunistic aggregations of lizards ( Farlow, 1976).
CONCLUSIONS
This study has made use of the limited available functional and ecological data to infer the likely maximum bite force of T. rex and to evaluate the maximum size of prey that this large theropod could have taken. The results obtained apply, by analogy, to other taxa as well. There are alternative methods for assessing bite force in extinct taxa, involving functional and phylogenetic approaches to the problem. These can be used to test the results derived here. Biomechanical analyses of teeth ( van Valkenburgh and Ruff, 1987; Farlow et al., 1991; Erickson et al., 1996), of the dentary ( Biknevicius and Ruff, 1992), and of the cranium ( Rayfield et al., 2001) can be used to develop tests of estimates of forces such as those arrived at in this study, increasing the rigor with which we can infer bite force in extinct taxa like T. rex . If it can be shown that these structures were unable to withstand forces ranging from 183,000 to 235,000 N, appropriately distributed along the length of the jaw, this would set a limit to inferences based on extrapolation from the biomechanics and behavior of living animals to the paleobiology of a long extinct organism.
Evidence of the bite force exerted by T. rex is consistent with either a predatory or a scavenging role for this animal. However, the analysis reported here, taken in conjunction with the comparison of predator and prey body masses, supports the hypothesis that T. rex could have been a competent, solitary predator even of large herbivores such as an adult Triceratops . Furthermore, relationships between predator and prey body masses among dinosaurs suggest that even sauropods weren't immune to the attacks of social groups of large theropods. The relationship between bite force and lethal capacity remains unclear at present. Likewise, the effects of bite force combined with forces imparted by struggling prey on the structure and function of the teeth, jaws and skull of T. rex have yet to be fully elucidated.
TABLE I Bilateral bite force and body mass data
| Taxa | Bite force (N) | Body mass ( kg) | Bite force source | Body mass source (if different from BF) |
|---|---|---|---|---|
| Vulpes vulpes —Red fox | 532.4 | 4.29 | Thomason, 1991 | Silva and Downing 1995 |
| Vulpes velox —Swift fox | 298.3 | 2.40 | Thomason, 1991 | Silva and Downing 1995 |
| Urocyon cineroargenteus —Grey fox | 350.7 | 3.76 | Thomason, 1991 | Silva and Downing 1995 |
| Canis latrans —Coyote | 1076.9 | 13.11 | Thomason, 1991 | Silva and Downing 1995 |
| Canis lupus —Wolf | 1412.2 | 31.60 | Thomason, 1991 | Silva and Downing 1995 |
| Crocuta crocuta— Spotted hyena | 9000.0 | 63.00 | Binder, pers. comm. | Kingdon, 1977 |
| Fells lybica —Wild cat | 369.3 | 4.17 | Thomason, 1991 | Silva and Downing 1995 |
| Felis Canadensis —Lynx | 767.8 | 9.77 | Thomason, 1991 | Silva and Downing 1995 |
| Felts concolor —Cougar | 1836.8 | 52.54 | Thomason, 1991 | Silva and Downing 1995 |
| Panthera pardus—Leopard | 2268.7 | 40.87 | Thomason, 1991 | Silva and Downing 1995 |
| Panthera let ) —Lion | 41675 | 163.40 | Thomason, 1991 | Silva and Downing 1995 |
| Chrysemys —Turtle | 50.0 | 0.92 | Sinclair and Alexander, 1987 | - |
| Pseudemys scripta —Turtle | 60.0 | 1.00 | Sinclair, 1983 | - |
| Ernys blandingii —Turtle | 65.0 | 0.99 | Sinclair, 1983 | - |
| Varanus —Varanid lizard | 40.0 | 2.60 | Sinclair and Alexander, 1987 | - |
| Caiman crocodilus — Caiman | 149.0 | 150 | Cleuren et al., 1995 | - |
| Alligator mississippiensis — Alligator | 18912.0 | 297.00 | Erickson, pers. Comm. | - |
| Tyrannosaurus rex | 253123.0 | 5371.00 | This study | Farlow et al., 1995 |
| Taxa omitted due to non-predatory behavior or other concerns | ||||
| Carcharhinus obscurus —Dusky shark* | 2892.0 | 213.30 | Snodgrass and Gilbert, 1967 | Kohler et al., 1995 |
| Didelphis virginiana —Possum* | 373.7 | 4.00 | Thomason, 1991 | Thomason et al., 1990 |
| Canis domesticus —Labrador retriever* | 1100.0 | 3070 | Strom and Holm, 1992 | American Kennel dub standard |
| Pongo pygmaeus —Orangutan * | 3424.0 | 56.60 | Lucas et aL, 1994 | Silva and Downing, 1995 |
| Homo sapiens —Human* | 1498.0 | 58.40 | van Eijden, 1991 | Ruff, 1994 |
| Caiman crocodilus —Caiman* | 74.0 | 1.00 | Sinclair and Alexander, 1987 | - |
| Caiman crocodilus — Caiman * | 100.0 | 1.30 | Sinclair, 1983 | - |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |