Uca (Paraleptuca) boninensis, Shih, Hsi-Te, Komai, Tomoyuki & Liu, Min-Yun, 2013
|
publication ID |
https://doi.org/10.11646/zootaxa.3746.1.8 |
|
publication LSID |
lsid:zoobank.org:pub:549F413B-9ED9-4A22-A5E2-40AA8AE0C35D |
|
DOI |
https://doi.org/10.5281/zenodo.5659434 |
|
persistent identifier |
https://treatment.plazi.org/id/FC601057-930E-FF9D-FF0F-FD7F670DFB1E |
|
treatment provided by |
Plazi |
|
scientific name |
Uca (Paraleptuca) boninensis |
| status |
sp. nov. |
Uca (Paraleptuca) boninensis View in CoL sp. nov.
[New Japanese name: Ogasawara-beni-shiomaneki] ( Figs. 2–7 View FIGURE 2 View FIGURE 3 View FIGURE 4. A – D View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Gelasimus lacteus . —Yoshiwara 1901: 314. Not Ocypode ( Gelasimus) lactea De Haan, 1835 .
Uca lactea .—Parisi 1918: 92 (part).—Takeda & Miyake 1976: 112 (list). Not Ocypode ( Gelasimus) lactea De Haan, 1835 .
Uca pulchella .—Parisi 1918: 93 (part).
Uca annulipes . —Balss 1922: 142.—Takeda & Miyake 1976: 112 (list). Not Gelasimus annulipes H. Milne Edwards, 1837 .
Gelasimus gaimardi —Sakai 1939: 617 (part), pl. 104(3), text-fig. 92 (part).—Sakai 1940: 47.
Uca (Amphiuca) chlorophthalmus crassipes .—Crane 1975: 102, 103 (part).—Sakai 1976: 606 (part).—Miyake 1983: 163 (part).
Uca gaimardi . —Imajima 1970: 188, pl. 6-4, fig. 4.—Ooishi 1970: 94, pl. XVI, fig. 3.—Shigei 1970: 53.—Takeda & Miyake 1976: 112.
Uca chlorophthalmus crassipes .—Takeda 1982: 208, 1 unnumbered fig. (part?).—Yoshigou 2001: 5–6 (part).—Kobayashi & Satake 2009: 215.
Uca chlorophthalmus .—Takeda 1995: 104 (part).
Uca crassipes .—Yoshigou 2002: 16, fig. 3, pl. 4(6).—Takeda & Ueshima 2006: 102 (part).—Government of Japan 2010: 66, 194.—Fujita & Naruse 2012: 215, unnumbered fig. (part).—Aoki & Wada 2013 (part).
Uca (Paraleptuca) annulipes .—Komatsu 2011: 277 (list). Not Gelasimus annulipes H. Milne Edwards, 1837 .
Uca (Paraleptuca) crassipes .—Komatsu 2011: 277 (list).
Uca (Paraleptuca) lactea . —Komatsu 2011: 277 (list). Not Ocypode ( Gelasimus) lactea De Haan, 1835 .
Uca ( Austruca) annulipes —Naderloo et al. 2010: 9 (part).
Material examined. Holotype: ♂ (CW 19.0 mm), Okumura River estuary, 30 May 1996, coll. K. Horikoshi, CBM-ZC 12094.
Paratypes: 9♂♂ (CW 11.0– 23.5 mm), 3 ♀♀ (CW 17.2–18.0 mm), 2 ovig. ♀♀ (CW 16.2, 18.8 mm), same data as holotype, CBM-ZC 11245; 1♂ (CW 19.6 mm), 2♀♀ (CW 17.7, 20.5 mm), same data as holotype, ZRC 2013.1747; 1♂ (CW 17.2 mm), 2♀♀ (CW 19.9, 20.6 mm), same data as holotype, NSMT-Cr 22390; 2♂♂ (CW 13.0, 17.3 mm), 3♀♀ (CW 14.3–17.3 mm), NCHUZOOL 13593, 13594, 13595, 13608, 13609, same locality as holotype, 19 October 2012, coll. T. Sasaki.
Comparative material. U. crassipes : 7♂♂ (CW 10.3–16.2 mm), 2♀♀ (CW 11.5, 13.5 mm), 1 ovig. ♀ (CW 15.1 mm), Hiyajo, Kume I., Ryukyu Is., 12 June 1995, coll. T. Komai, CBM-ZC 3062; 2♂♂ (CW 16.1, 17.5 mm), estuary of Miyara River, Ishigaki I., Yaeyama Is., 11 July 2003, coll. Y. Matsuzawa, CBM-ZC 7460; 2♂♂ (CW 16.3, 16.6 mm), estuary of Yanshuei River, Tainan, Taiwan, 4 August 2009, coll. J.-H. Lee, CBM-ZC 11958 (ex NCHUZOOL 13472); 2 ♀♀ (CW 14.1, 16.0 mm), Hengchun Peninsula, Pingtung, Taiwan, 20 August 2009, coll. J.-H Lee, CBM-ZC 11958 (ex NCHUZOOL 13493).
Uca splendida : 2 ♂♂ (CW 17.0, 19.0 mm), Citou, Penghu Is., Taiwan, 23 May 2008, CBM-ZC 11959 (ex NCHUZOOL 13486); 2 ♀♀ (CW 12.5, 14.9 mm), same locality, 19 August 2009, coll. H.-T. Shih et al., CBM-ZC 11960 (ex NCHUZOOL 13485).
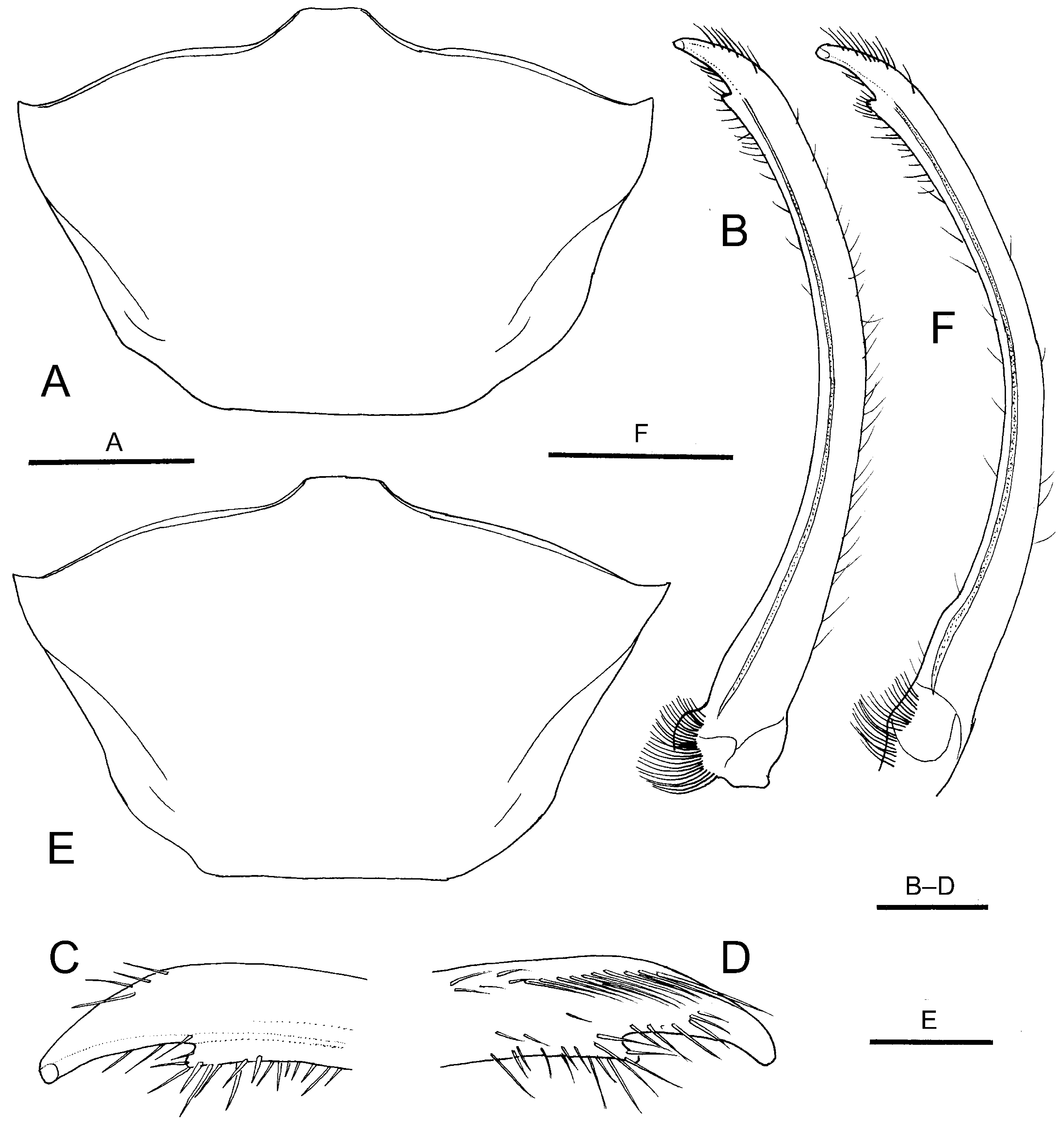
Diagnosis. Male. Carapace ( Figs. 2 View FIGURE 2 , 4A View FIGURE 4. A – D , 5 View FIGURE 5 A, 6A, C, 7) 1.5–1.6 times as wide as long. Front ( Fig. 3 View FIGURE 3 A) wide, subtrapezoidal in general outline, not constricted. Orbits slightly oblique in dorsal view; upper orbital margin doubled; lower orbital margin with row of subrectangular to rounded granules increasing in size laterally ( Fig. 3 View FIGURE 3 A). Anterolateral angles (= external orbital angle) subacute to blunt, produced anteriorly. Anterolateral margins clearly delimited, relatively long, slightly diverging anteriorly, turning at weak angle into well-delimited, slightly sinuous dorsolateral margins; one posterolateral stria. Major cheliped ( Fig. 3 View FIGURE 3 B) with shallow, triangular depression on outer surface of palm slightly proximal to base of pollex; tip of pollex with small subdistal tooth, sometimes irregularly bifid; palm finely granular. Ambulatory meri moderately broad. G1 ( Fig. 4B–D View FIGURE 4. A – D ) relatively stouter than in U. crassipes ( Fig. 4 View FIGURE 4. A – D F); terminal process gradually tapering distally, without flanges, tubular; subterminal process (= thumb) short, rounded.
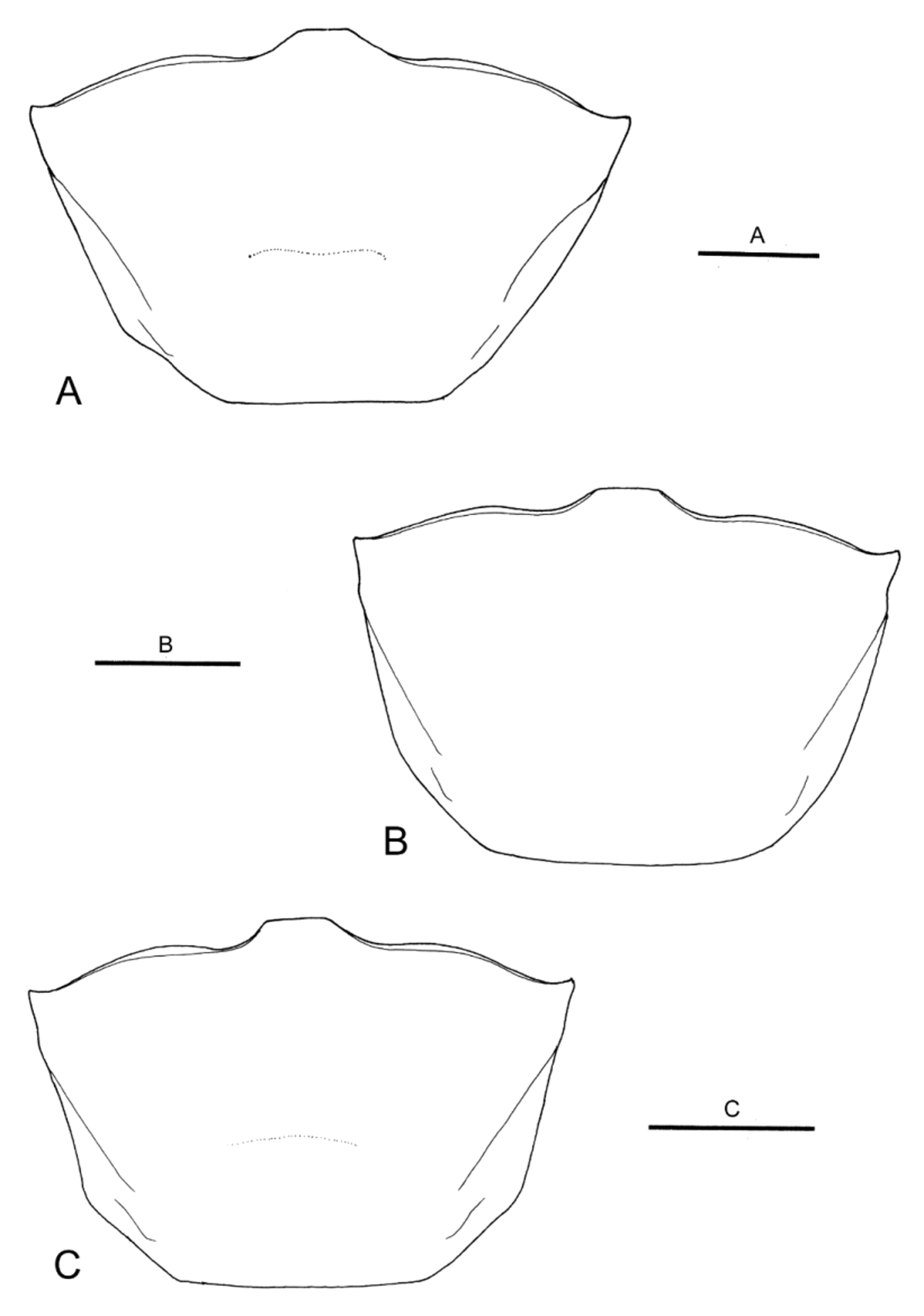
Female. Carapace ( Fig. 5 View FIGURE 5 B) 1.4–1.5 times as wide as long. Anterolateral angles less acute than in males, produced anteriorly. Anterolateral margins slightly diverging anteriorly, slightly sinuous, turning at weak angle into nearly straight dorsolateral margins, relatively less diverging anteriorly.
Size. Largest male CW 23.5 mm; largest female CW 20.5 mm.
Variation. As is apparent from the Diagnosis above, the shape of the carapace is sexually dimorphic. Furthermore, the anterolateral to dorsolateral margin of the carapace is less sinuous and more diverging anteriorly in the largest male paratype (CW 23.5 mm, CBM-ZC 11245; Fig. 5 View FIGURE 5 A) than in the holotype and other male paratypes, approaching the condition seen in U. crassipes ( Fig. 7 View FIGURE 7 ).
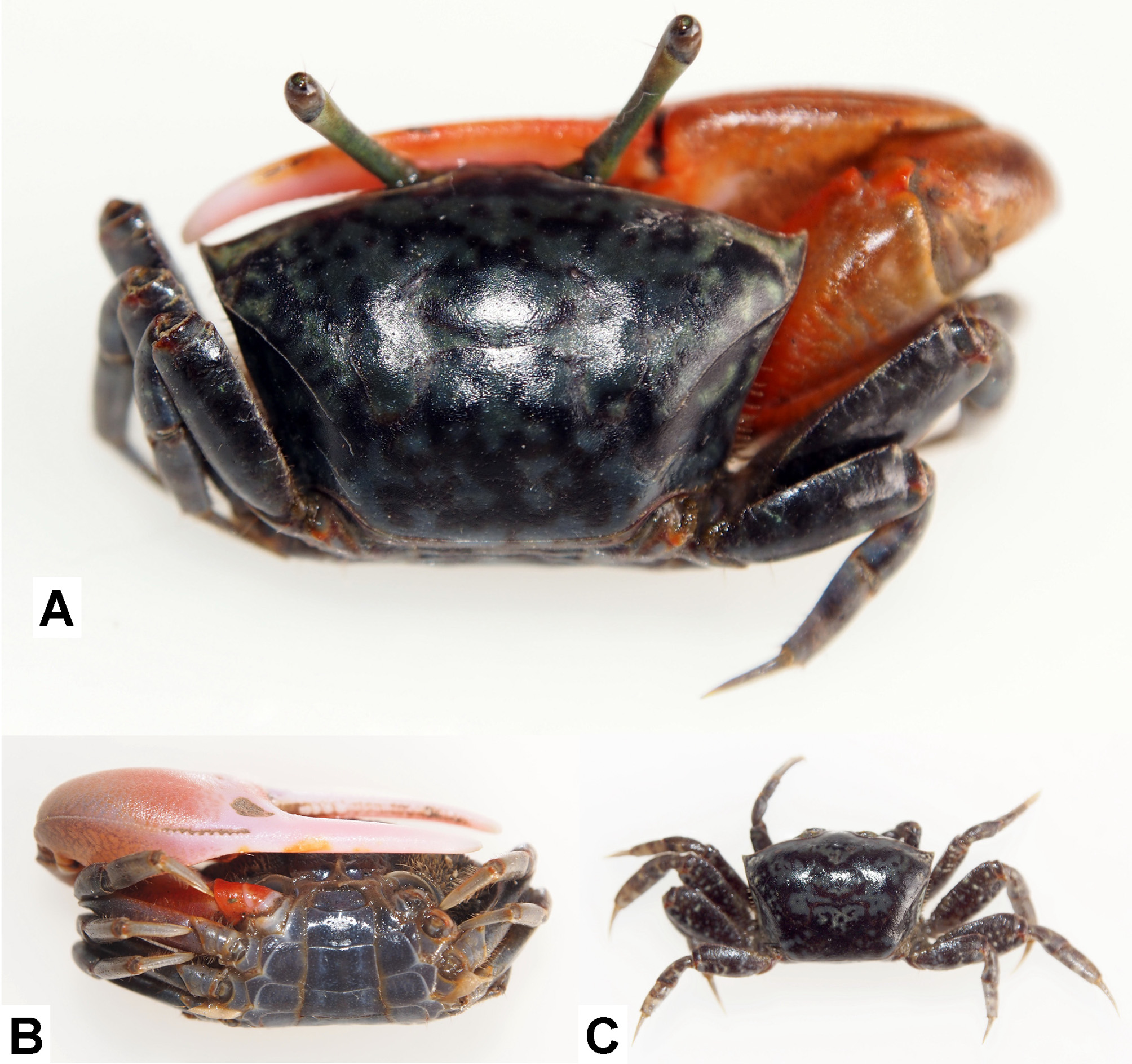
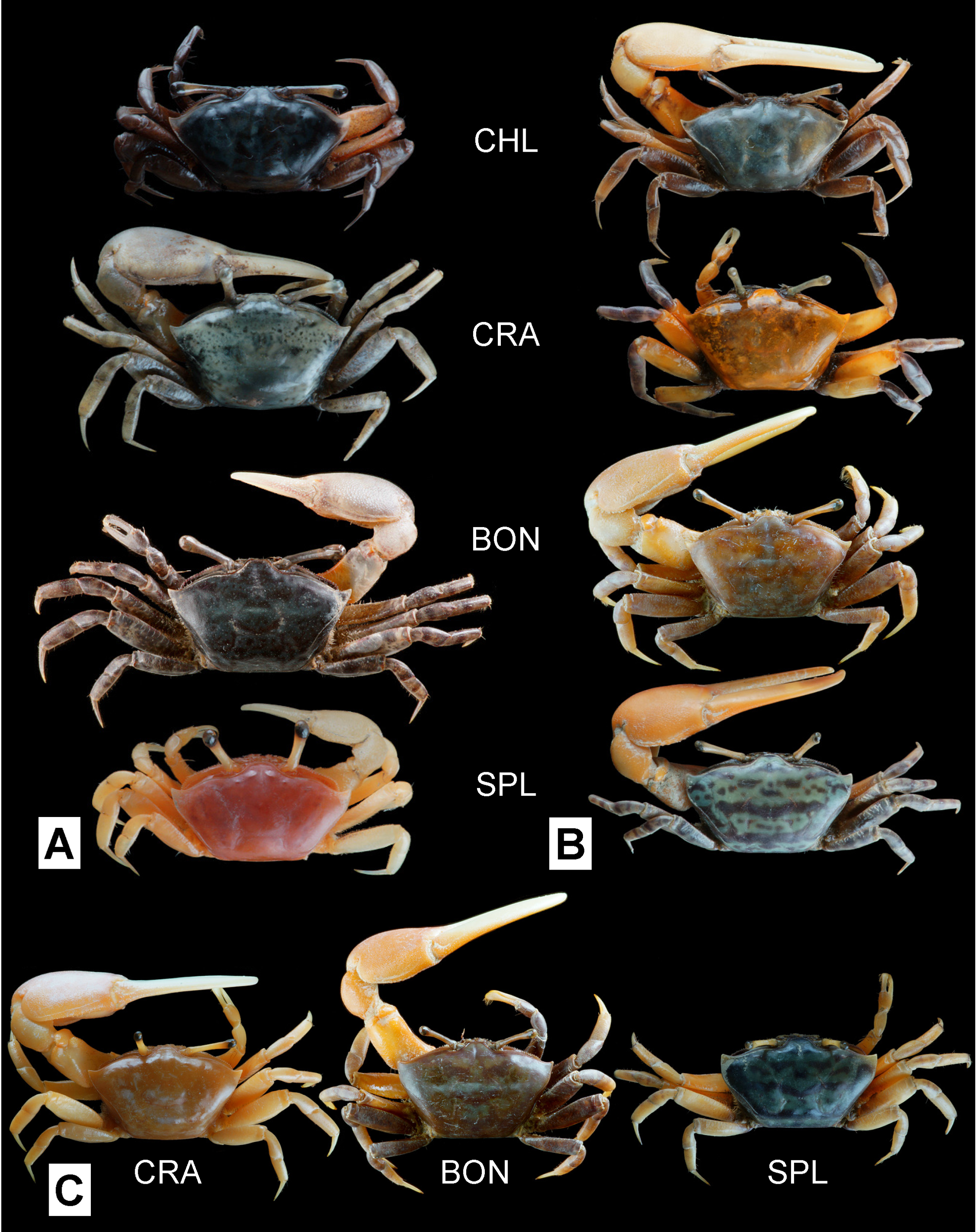
Coloration ( Fig. 6 View FIGURE 6 ). Carapace considerably variable in coloration, but dorsal surface mottled with black and blue, gray or white in many male specimens. Anterior part of carapace sometimes orange-red in females. Carapace of juveniles cream-yellow or pale green with mottled brown. Eyestalks olive to grayish. Male major chela rose pink to scarlet red, pollex and dactyl pale pink to white; merus reddish. Male minor cheliped, female chelipeds, and ambulatory legs mottled dark brown or dark gray, occasionally with red patch on meri.
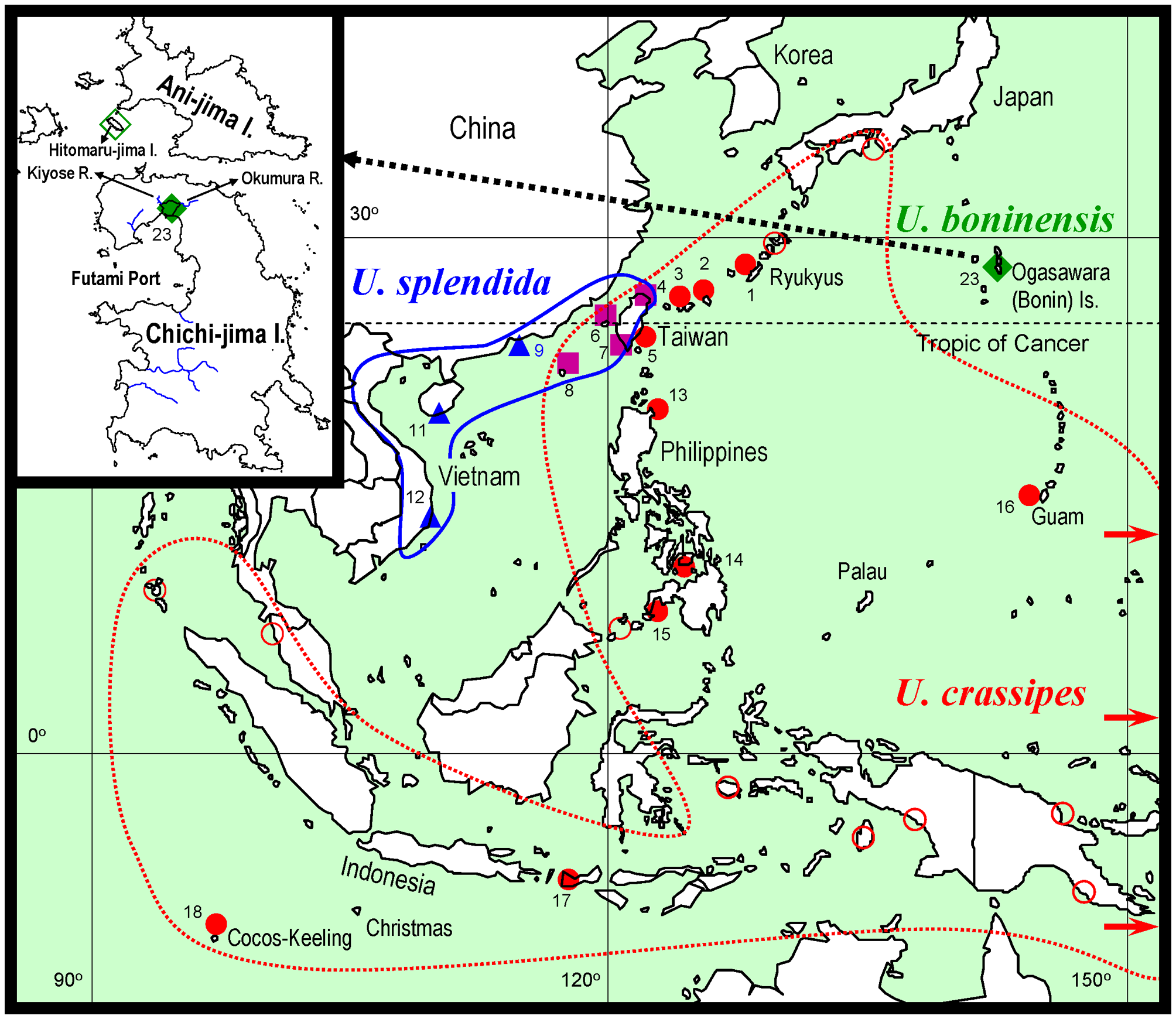
Ecology. Uca boninensis sp. nov. is found on the upper intertidal zone of estuaries of the small rivers, Okumura and Kiyose rivers (insert in Fig. 1 View FIGURE 1 ), where the substratum is composed of rock fragments and muddy sand. Burrows have openings of 1–2 cm in diameter and are not deeper than 20 cm, often limited by rock fragments beneath.
Morphological comparison. Uca boninensis sp. nov. is very similar in morphology to U. crassipes , and differentiation based on morphological characters is not easy. The anterolateral carapace margins of U. boninensis sp. nov. nevertheless seem to be less diverging, and have less acute external orbital angles than in U. crassipes (cf. Fig. 4A View FIGURE 4. A – D and Fig. 4 View FIGURE 4. A – D E; Fig. 7 View FIGURE 7 ). As noted by Yoshigou (2002), the G1 is relatively stouter in U. boninensis sp. nov. than in U. crassipes (cf. Fig. 4B View FIGURE 4. A – D and Fig. 4 View FIGURE 4. A – D F). The dorsolateral margins of the carapace may be slightly less divergent anteriorly in the females of U. boninensis sp. nov. than in U. crassipes (cf. Fig. 5 View FIGURE 5 B and Fig. 5 View FIGURE 5 C).
Uca splendida differs from U. boninensis sp. nov. in the subparallel anterolateral margins of its carapace and the distally more truncate terminal process of the G1 (cf. Shih et al. 2012: figs. 2B, C, 7).
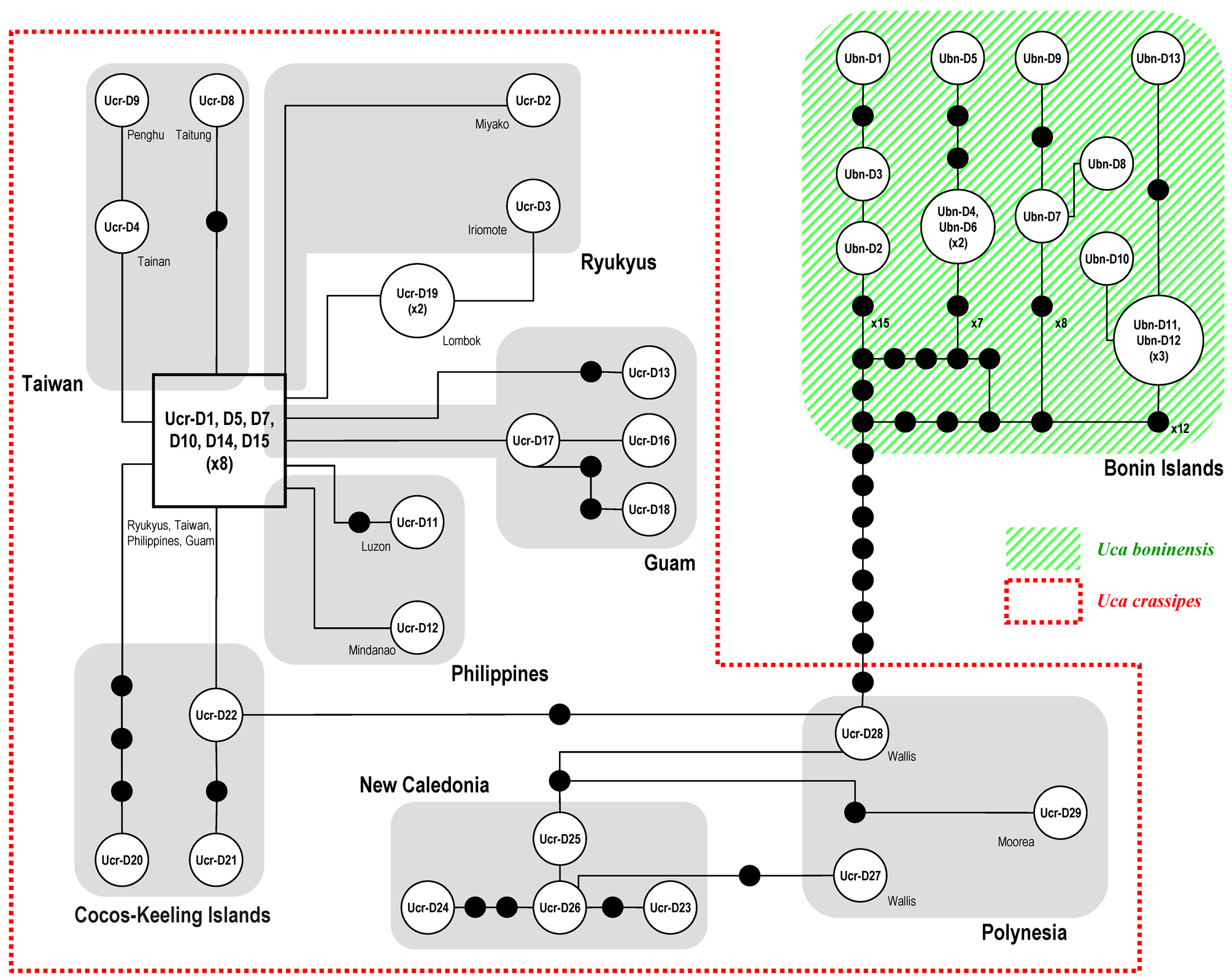
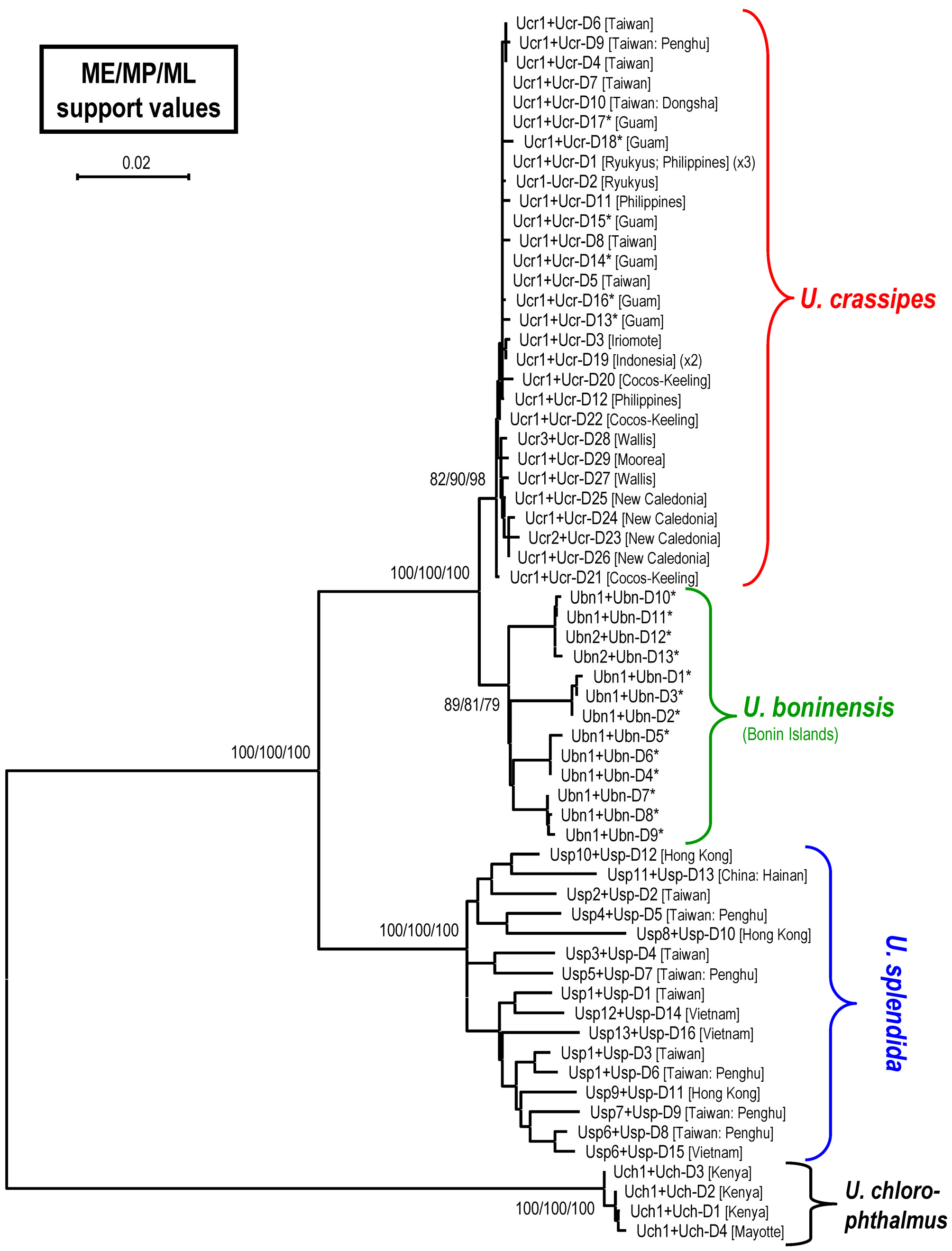
DNA analysis. A total of five specimens of U. boninensis sp. nov., 28 U. crassipes , 16 U. splendida and 4 U. chlorophthalmus specimens were used in the molecular phylogenetic analysis. A 658-bp segment of COI was amplified, resulting in 19 different haplotypes (Table 1). The studied segment of the COI sequences was AT-rich (61.8%) (T, 32.2%; A, 29.6%; G, 16.8%; C, 21.4%). In this gene fragment, 70 positions were variable and 23 were parsimoniously informative. For CR, a 962 bp segment after alignment was compared, resulting in 62 different haplotypes (including the heteroplasmic ones; Table 1). The CR segment was also AT-rich (78.6%) (T: 34.7%, A: 44.0%, G: 8.0%, C: 13.4%). In this gene, 341 positions were variable and 282 were parsimony informative. The phylogram of ME analysis, with the bootstrap values from the ME, MP and ML analyses, are shown in Figure 8 View FIGURE 8. A . Only values greater than 50% are shown. In the MP analysis, 28 trees were recovered with a tree length of 670 steps, a consistency index of 0.66, and a retention index of 0.91. The haplotypes of COI or CR of U. boninensis sp. nov. are not shared by other species.
According to the phylogenetic tree ( Fig. 8 View FIGURE 8. A ), U. crassipes , U. boninensis sp. nov. and U. splendida form a major clade, with the former two as sister species. The monophylies of U. crassipes and U. boninensis sp. nov. have medium to high supports (82%–98%, and 79%–89%, respectively).
The pairwise nucleotide divergences for COI and CR with K2P distance between species are shown in Table 2 View TABLE 2 . It is apparent that the COI divergence between U. crassipes and U. boninensis sp. nov. is minor (mean 0.28%). There is no diagnostic nucleotide difference between the two species. On the other hand, the CR divergence between these two sister species is higher than the COI value, but still lower compared with interspecific divergence between other species. Although the average divergence between the two sister species (3.18%) is higher than the average intraspecific values of U. crassipes and U. boninensis sp. nov. (0.38% and 2.56%, respectively), the highest intraspecific value (3.97%) of U. boninensis sp. nov. is higher than the lowest interspecific value (2.45%). There are nevertheless 11 nucleotide differences (based on the realigned 938-bp segment) always separate the two sister species, with the diagnostic genetic distance being 1.17%.
The CR haplotype network constructed depicts the relationships among the haplotypes of clades of U. crassipes and U. boninensis sp. nov. ( Fig. 9 View FIGURE 9 ). The haplotypes (Ucr-D1, -D5, -D7, -D10, -D14 and -D15) from the Ryukyu Is., Taiwan, Philippines and Guam are more central relative to other haplotypes, and are therefore assumed to represent the ancestral haplotypes (cf. Clement et al. 2000; Avise 2009). There are at least 22 steps separating the two species. The haplotype Ucr-D28 from Wallis I. in the South Pacific is closest to the U. boninensis haplotypes.
Intraspecific Interspecific
COI CR U. crassipes U. boninensis U. splendida U. chlorophthalmus
U. crassipes 0.02 0.38 — 3.18 11.01 23.15
(0–0.3) (0–1.21) (2.45–4.08) (9.5–12.58) (22.53–23.83) Remarks. The taxa of Uca from the Ogasawara Is. have been reported under various names (see synonymy list), but this reflects the confused state of the taxonomy of the genus prior to Crane (1975). Only one species has thus far been confirmed from the Ogasawara Is. (cf. Yoshigou 2002; Kobayashi & Satake 2009), U. boninensis sp. nov. In fact, Takeda & Ueshima (2006) referred specimens collected at Kiyose, Chichi-jima I. ( 12 males, 2 females, 3 February 1894, UMUTZ-Crs-Dec-Bra-539), originally identified with Gelasimus annulipes var. lacteus , to U. crassipes . All previous records of fiddler crab from the Ogasawara Is. are consequently referred to U. boninensis sp. nov.
Etymology. The species is named after the Bonin Is., the old name for the Ogasawara Is., where the new species was discovered. "Bonin" is derived from the Japanese munin, meaning "a place without residence."
Distribution. The specimens examined came from Okumura River estuary of Chichi-jima I., Ogasawara Is., but the new species also occurs in estuary of Kiyose River (Takeda & Ueshima 2006, as U. crassipes ; K. Horikoshi, pers. com.; T. Komai, pers. obs.; insert in Fig. 1 View FIGURE 1 ). Previous records referable to U. boninensis sp. nov. (see synonymy list) from the Ogasawara Is. are mostly from Chichi-jima I. Ooishi (1970) reported on the occurrence of a species of Uca under the name U. crassipes from rocky shores on Hitomaru-jima I., a small island close to Ani-jima I. (insert in Fig. 1 View FIGURE 1 ). It is likely that U. boninensis has also colonized other small islands of the Ogasawara Is.
TABLE 2. Matrix of percentage pairwise nucleotide divergences with K 2 P distance based on cytochrome oxidase I (COI) (lower left) and control region (CR) (upper right) within and between Uca crassipes (White, 1947), U. boninensis sp. nov., U. splendida (Stimpson, 1858) and U. chlorophthalmus (H. Milne Edwards, 1837). Values of range are shown in parentheses.
| U. boninensis | 0.06 (0–0.15) | 2.56 (0–3.97) | 0.28 (0.15–0.46) | — | 11.8 24.92 (9.52–13.78) (23.78–26.41) |
|---|---|---|---|---|---|
| U. splendida | 0.79 (0–1.86) | 4.75 (1.21–6.68) | 2.78 (2.49–3.46) | 2.74 (2.33–3.3) | — 24.22 (22.53–25.6) |
| U. chlorophthalmus | 0 | 0.27 (0.11–0.43) | 6.46 (6.45–6.62) | 6.45 (6.45–6.45) | 7.21 — (6.97–7.67) |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Brachyura |
|
Family |
|
|
Genus |
|
|
SubGenus |
Paraleptuca |